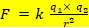Coulomb’s Law and Periodic trends- Force of attraction between oppositely charged particles
Coulomb’s law can be used for qualitative comparison of attractions among the charged particles in molecules, ions and within the atom etc. Here q1 and q2 are the charges on the particles and r is the distance of separation between the charged particles.
Qualitative meaning of Coulomb’s Law and Periodic trends.
- The electrostatic force of attraction is directly proportional to the product of the charges on the interacting particles. This means that the greater the magnitude of the charges, the stronger the force of attraction between them.
- Examples: NaCl vs MgO:
- Na⁺ and Cl⁻: Charges = +1 and –1 → Product = 1
- Mg²⁺ and O²⁻: Charges = +2 and –2 → Product = 4
→ MgO has 4 times stronger attraction than NaCl (assuming equal distance), leading to higher lattice enthalpy and higher melting point. - Ionization Energy Trends: In atoms with a +2 nucleus (He) vs +1 nucleus (H), the attraction to electrons is stronger in He, making it harder to remove an electron despite nearly similar size.
- The electrostatic force of attraction between charged particles is inversely proportional to the square of the distance between them. This means that as the distance between the particles increases, the force of attraction decreases rapidly. Therefore, more energy is required to separate charges that are closer together, because the attractive force between them is stronger.
Examples:
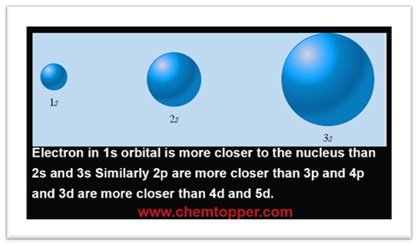
As the shell number increases, the distance of the orbitals (and electrons) from the nucleus also increases. As a result, the electrostatic attraction between the nucleus and the electron decreases, leading to an increase in the energy of the electron in higher shells.
Electrons in the first energy level (closer to the nucleus) experience a much stronger attraction to the positively charged nucleus than electrons in outer shells. As a result, more ionization energy is needed to remove an electron from the first shell than from higher shells.
Electrons in different shells of an atom:
- In ionic solids like NaCl, the positive (Na⁺) and negative (Cl⁻) ions are held together by electrostatic forces of attraction in a closely packed lattice. In compounds like MgO, the ions—Mg²⁺ and O²⁻—carry higher charges and are smaller in size, resulting in even stronger electrostatic attraction and a more tightly packed lattice compared to NaCl. This leads to a much higher lattice enthalpy in MgO, making it more thermally stable and requiring significantly more energy to separate the ions.
- For example:
- MgO has a much higher melting point (~2852 °C) than NaCl (~801 °C), which correlates with its higher lattice enthalpy.
- In short, lattice enthalpy is directly related to melting point in ionic compounds—Higher charges and smaller size =stronger attractions = higher lattice energy = higher m.p.t.
Mantra in chemistry-Force of attraction ∞ Energy
Explanation of the Flow:
- More force of attraction
→ Typically due to higher effective nuclear charge or smaller atomic size. - Exothermic process
→ When electrons are added or bonds are formed, energy is released. - Less energy (more negative energy)
→ Indicates a more stable state (like in electron affinity or bond formation). - More stability
→ Atoms reach low-energy configurations, like noble gases. - Less reactivity
→ Stable atoms (e.g., noble gases) are less likely to react.
Coulomb’s Law and Periodic trends-Where This Applies:
- Electron Affinity: More attraction → more energy released (exothermic) → more stable anion → less likely to gain another electron.
- Ionization Energy: Opposite—more attraction means more energy is required to remove an electron.
- Covalent Bond Formation: Atoms bond to reach lower energy (stability)→ Small atoms means small bonds → more attraction →stronger bond →more energy needed to break it →High bond energy.
- Hydration enthalpy – more charge and small size means more stronger hydration by water molecules and more negative hydration enthalpy. (Look at the picture in flash card)
- Lattice enthalpy and melting points-more charge and small size means more stronger attraction →stronger lattice and higher melting point→ stable compound.