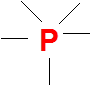
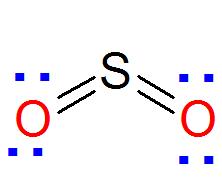
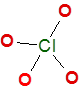
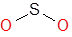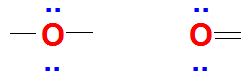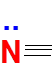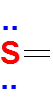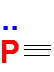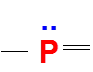Understanding the Importance of Lewis Dot Structures
To begin with, Lewis dot structure is a classical bonding model that uses only the valence electrons of atoms. It plays a foundational role in chemistry because it connects to many important concepts. For example, Lewis structures are essential for understanding chemical bonding, resonance, valence shell electron pair repulsion (VSEPR) theory, molecular polarity prediction, and even the mechanisms of chemical reactions. Therefore, it is crucial to learn how to draw Lewis dot structures accurately for atoms, ions, molecules, polyatomic ions, and ionic compounds.
Learning Lewis Structures Step-by-Step
Fortunately, you can learn to create correct Lewis dot structures by following four simple steps. These are designed to be easy to understand and practical to apply. However, it is important not to skip or rearrange them, especially during the learning process. Each step builds upon the previous one, so completing them in order ensures you fully grasp the method. Once you master the technique, you will be able to draw the Lewis structure of any chemical species quickly and with confidence.
Key Concepts to Watch For
Along the way, you will encounter important terms such as valence electrons, electronegativity, stable electronic configuration, formal charges, bonding pairs, lone pairs, and the types of bonds—single, double, and triple bonds. If you’re not familiar with these yet, don’t worry. Each term will be explained clearly during the steps, so you will understand how and why they matter in the context of Lewis structures.
STEP 1 : COUNT THE TOTAL VALENCE ELECTRONS.
What Are Lewis Dot Structures and Why Do Valence Electrons Matter?
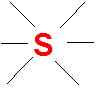
In a Lewis dot structure, we use only the valence electrons to represent how atoms bond and interact. These are the electrons found in the outermost shell of an atom’s electron configuration, and they play a central role in determining chemical reactivity and bonding behavior.
To put it simply, valence electrons are the ones involved in forming chemical bonds—whether ionic or covalent. That’s why understanding their placement is key to drawing accurate Lewis structures.
For instance, consider the example below. It will help illustrate how valence electrons are represented and used in a Lewis dot structure.
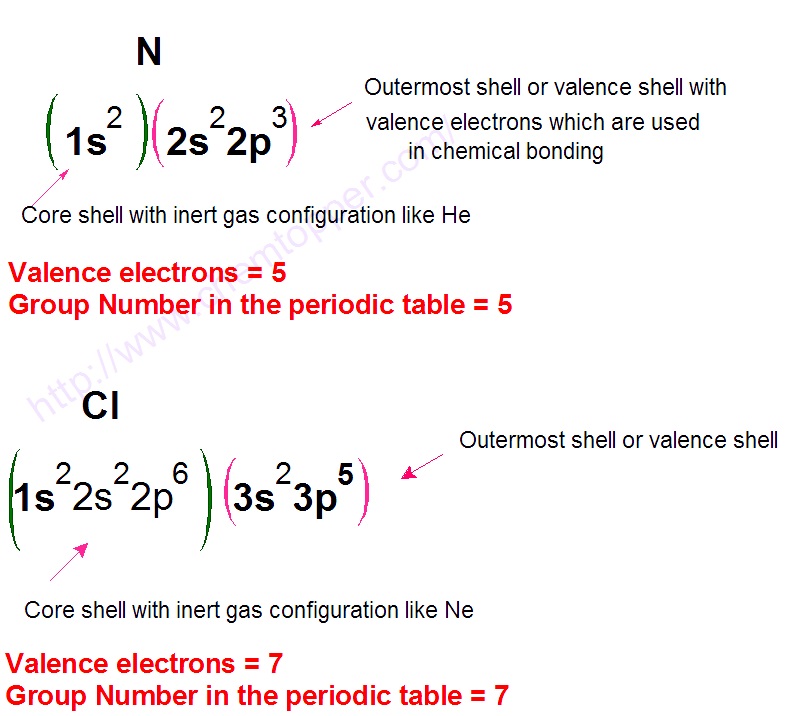
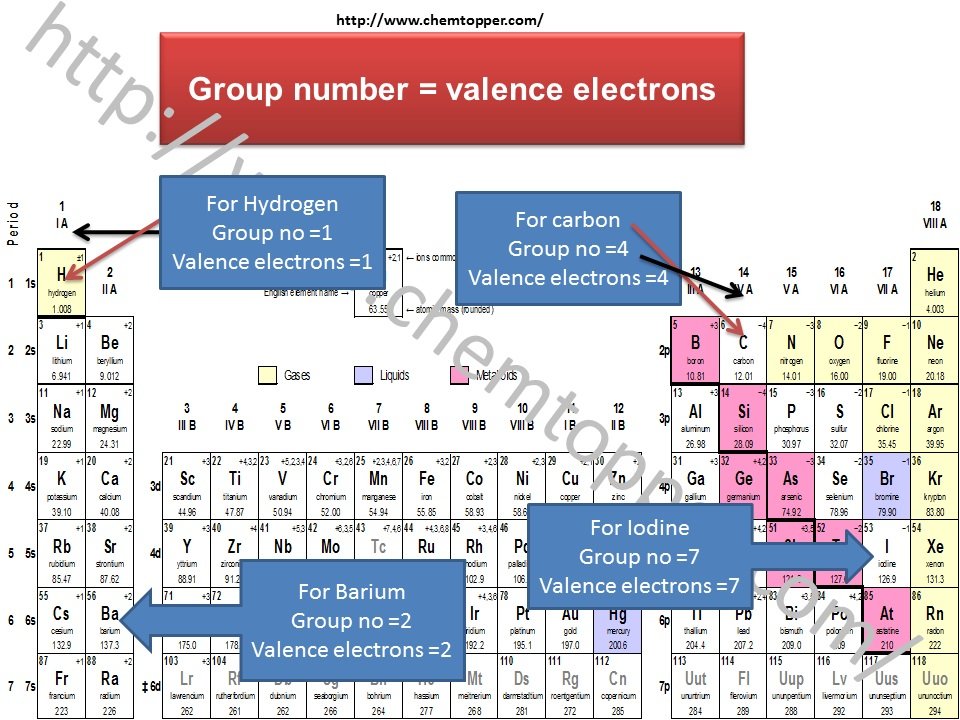
How to Find Valence Electrons Using the Periodic Table
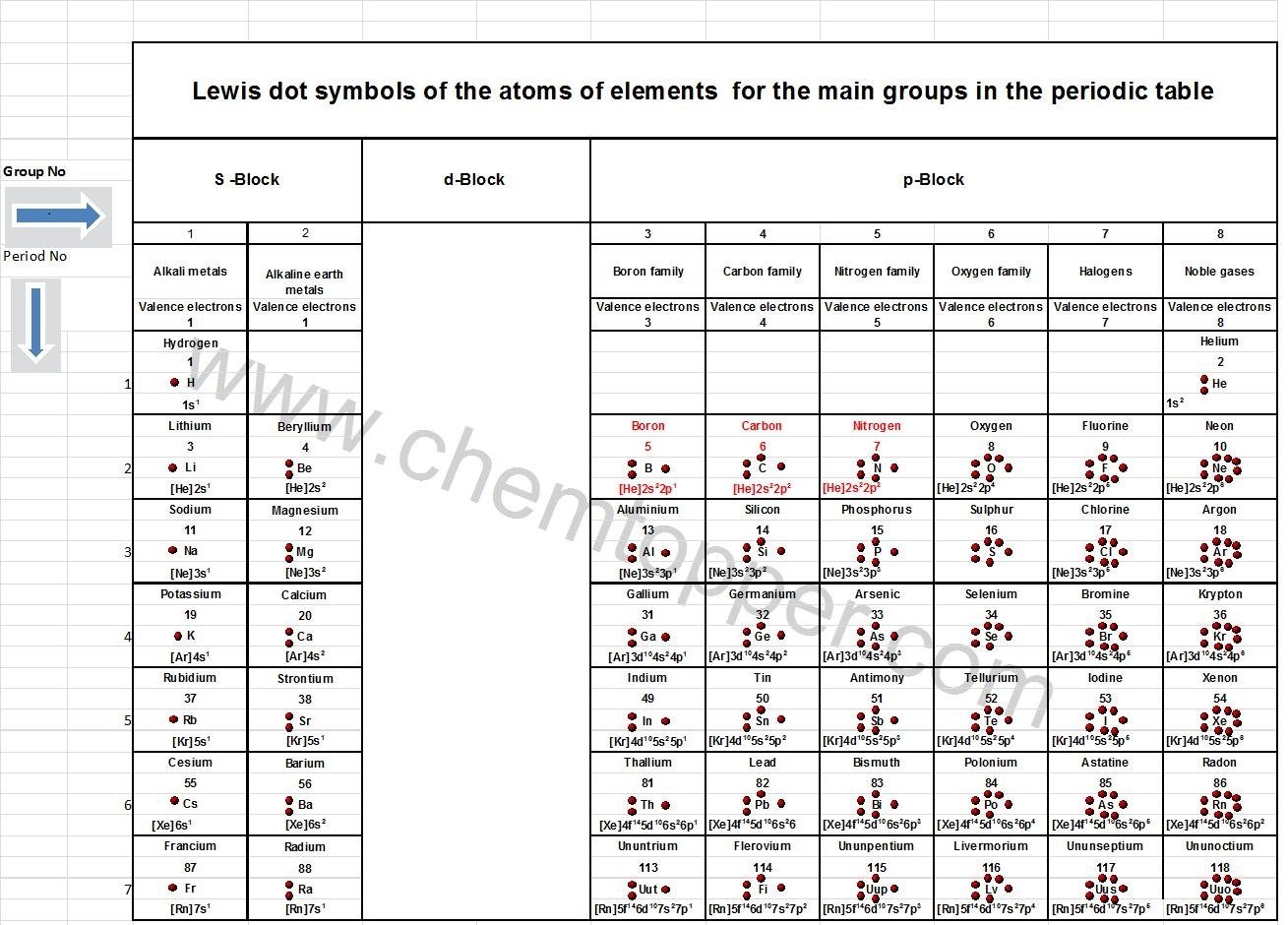
If you’re not comfortable writing out full electronic configurations, don’t worry—there’s a much easier way to determine the number of valence electrons: just use the periodic table. In most cases, the number of valence electrons is the same as the group number (for main group elements). For example:
-
Oxygen is in Group 16, so it has 6 valence electrons.
-
Beryllium is in Group 2, so it has 2 valence electrons.
-
Nitrogen, found in Group 15, has 5 valence electrons.
-
Chlorine, in Group 17, has 7 valence electrons.
As you can see, this method is quick, reliable, and perfect for building Lewis dot structures without getting into complex configurations.
That’s your first step—simple and effective!
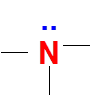
Lewis dot structure of N atom
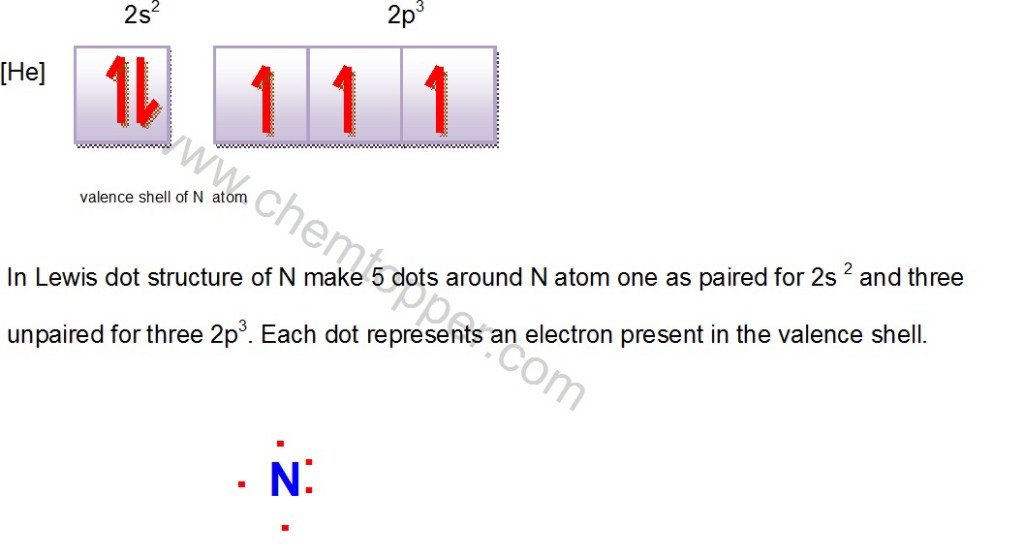
Let’s do one more example:
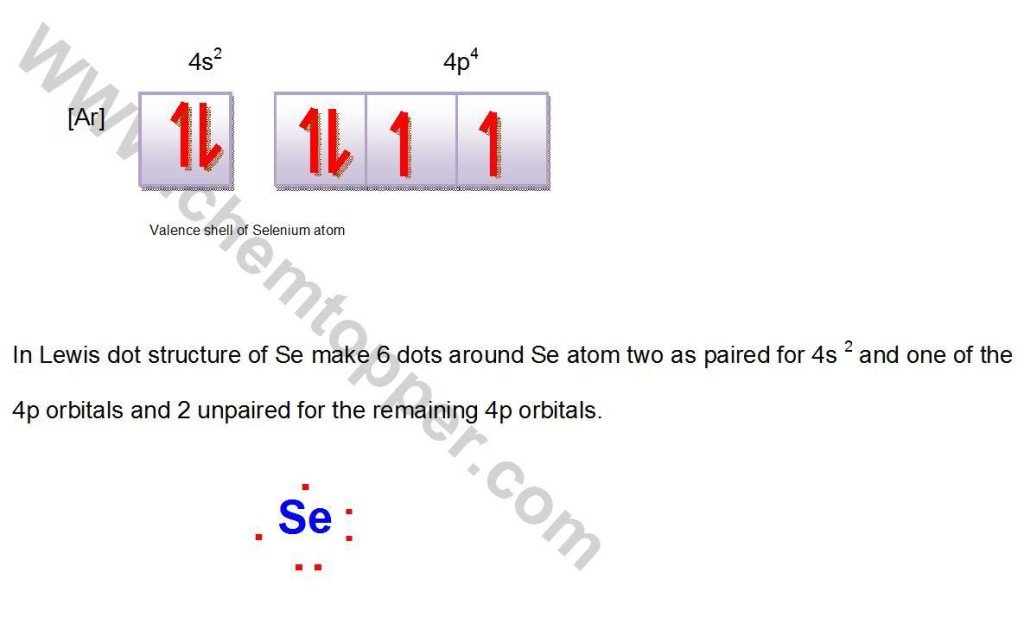
Lewis dot structure of Se atom
Se atom
Electronic configuration:
[Ar]3d104s24p4
Valence shell is 4s24p4 with total 6 electrons.
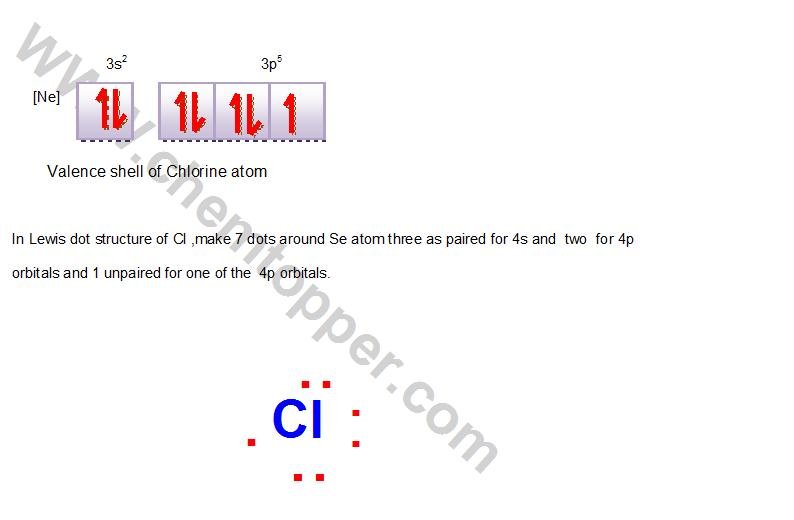
Lewis dot structure of Monoatomic ions:
How Charges Affect Valence Electrons in Ions
When atoms become ions, they do so by either gaining or losing electrons. This change directly affects the total number of valence electrons used in their Lewis dot structure.
To understand this better, consider the following:
-
If an ion has a negative charge, it means the atom has gained electrons equal to the magnitude of the charge.
-
On the other hand, if the ion has a positive charge, it means the atom has lost electrons.
Therefore, you must add or subtract electrons from the valence electrons of the neutral atom, depending on the charge, before drawing the Lewis structure.
For example, let’s look at the nitride ion (N³⁻). Nitrogen normally has 5 valence electrons (Group 15). However, a 3⁻ charge means it has gained three extra electrons, making a total of 8 valence electrons. This is equivalent to the electron configuration of neon (10 electrons in total).
As a result, the Lewis structure of N³⁻ will show 8 valence electrons around the nitrogen symbol, along with the 3⁻ charge indicated outside the brackets.
[He]2s22p6
Valence electrons are 8 (2 in 2s and 6 in 2p)

Now let us try Lewis dot structure of Sulfide ion ( S2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16).
[Ne]4s24p6
Valence electrons are 8 (2 in 3s and 6 in 3p)
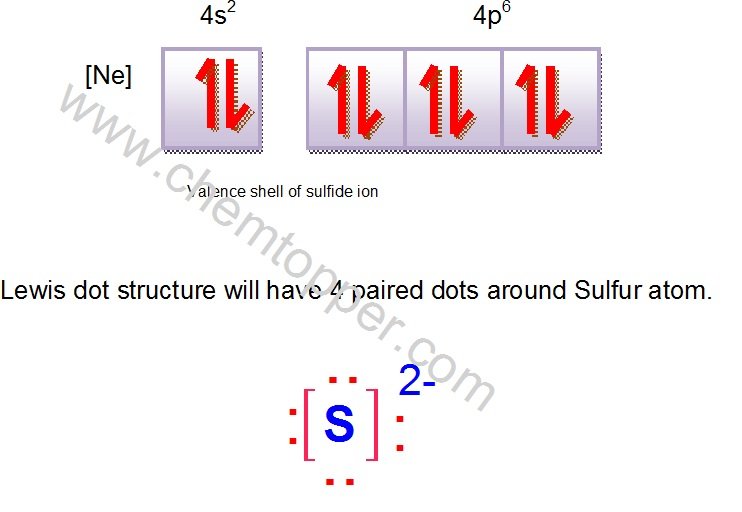
Lewis dot structure will have 4 paired dots around Sulfur atom.For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure.
Lewis dot structures for Polyatomic ions and molecules :
However for molecules and polyatomic ions we need to consider many more factors before drawing a correct Lewis dot structure. Let’s practice step one “count the total valence electrons’ on molecules and polyatomic ions.
Molecule:
SO2 (Sulfur dioxide)
S is in the 6th group and O is also in the same group in the periodic table.
Total valence electrons = 6(S) + 2*6(2O) = 6+12=18
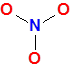
Ion:
NO3– (nitrate ion)
Total valence electrons = 5(N) + 3*6(3O) +1 (-1 charge) = 5+18+1=24

STEP 2 : MAKE A SKELETON OF THE STRUCTURE
How to Choose the Central Atom Using Electronegativity Trends
To begin with, choosing the correct central atom in a Lewis structure requires a solid understanding of electronegativity and its trends across the periodic table.
SELECT LEAST ELECTRO-NEGATIVE (EN) ATOM AS THE CENTRAL ATOM AND MAKE A SKELETON OF THE STRUCTURE WITH REST OF THE ATOMS AROUND IT
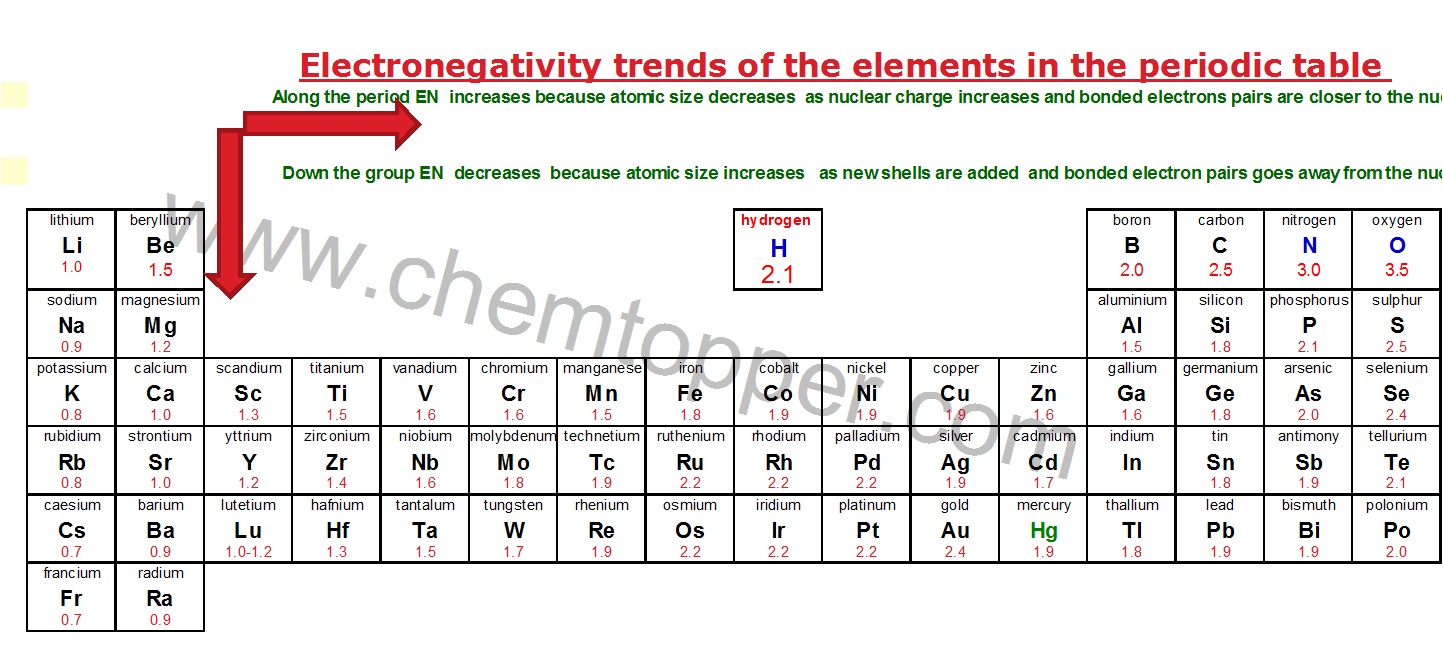
Here is a table that depicts electronegativity trends in the periodic table
Now let us select least EN atom as the central atom in our molecule SO2.You can use the periodic table while deciding about it. S is placed below O in the periodic table and hence it is bigger in size and less EN than O.
SO2 S is the central atom because S is less EN then O
In the skeleton of the molecule two oxygen atoms making single bonds with S
NO3– N is the central atom because N is less EN then O .In the skeleton of the ion three O atoms making three single bonds with central atom N.
Key Rules to Remember When Choosing the Central Atom
When drawing a Lewis structure, keep the following important rules in mind:
1. The central atom must be capable of forming more than one single bond.
In most cases, the central atom needs to bond with multiple surrounding atoms. Therefore, it should have enough valence electrons and available orbitals to accommodate more than one bond.
2. Hydrogen and Helium are exceptions.
These Group 1 elements can never have more than 2 electrons because their electronic configuration includes only the 1s orbital.
As a result, they can form only one bond and are never placed at the center of a Lewis structure.
3. Second-period elements have a strict octet.
Elements like carbon, nitrogen, oxygen, and fluorine can accommodate a maximum of 8 electrons in their valence shell.
This limitation occurs because they lack empty d orbitals.
Therefore, they cannot form expanded octets, and you must avoid assigning more than 8 electrons to them in a structure.
4. Third-period and heavier elements can expand the octet.
Starting from the third period, elements have available d orbitals, allowing them to hold more than 8 electrons.
Consequently, atoms like phosphorus, sulfur, and chlorine can form expanded octet structures when needed.
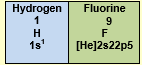
5. Hydrogen and Fluorine are never central atoms.
Both require only one electron to complete their respective duplet (H) and octet (F).
Therefore, they typically form only one single bond and are always placed on the outside of the structure.
STEP 3 : COMPLETE THE OCTET.
COMPLETE THE OCTET OF THE MOST ELECTRONEGATIVE ATOM WITH MINIMUM FORMAL CHARGES
Understanding Formal Charge in Lewis Structures
When drawing Lewis structures, it’s important to determine the formal charge on each atom. This helps assess whether the structure is valid and stable.
To begin with, formal charge is the charge assigned to an atom based on the difference between the number of valence electrons it owns in its neutral state and the electrons it uses in the structure.
What Is Formal Charge?
In simple terms, formal charge tells us whether an atom has gained or lost control over its electrons during bonding.
We use the formula:
Formal Charge (FC)=Valence electrons−(Bonding electrons2)−Lone pair electrons\text{Formal Charge (FC)} = \text{Valence electrons} – \left( \frac{\text{Bonding electrons}}{2} \right) – \text{Lone pair electrons}
Alternatively, since each covalent bond represents one shared electron from the atom, and each lone pair includes two electrons, we can rewrite the formula as:
FC=Group Number−Number of Bonds−2×Number of Lone Pairs\text{FC} = \text{Group Number} – \text{Number of Bonds} – 2 \times \text{Number of Lone Pairs}
How Does It Work?
Let’s break it down:
-
Valence electrons correspond to the atom’s group number in the periodic table.
-
Each bond counts as one electron “contributed” by the atom.
-
Each lone pair counts as two electrons.
As a result, if an atom ends up with more electrons around it than it originally had, it gains a formal negative charge.
On the other hand, if it has fewer electrons, it gets a formal positive charge.
Why Is Formal Charge Important?
Therefore, calculating formal charge helps determine the most stable Lewis structure among several possibilities. A structure where formal charges are minimized—or where negative charges reside on more electronegative atoms—is usually the most correct representation of the molecule or ion.
Example: Oxygen (O),Nitrogen (N),Carbon (C)
It has 6 valence electrons so it is very happy with two bonds and two lone pairs in the Lewis dot structures
No of bonds = 2
Lone pairs = 2
FC = 6-2-(2*2) =0
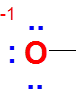
However if Oxygen has one bond with three lone pairs in Lewis dot structure, then
Valence electrons of O = 6
No of bonds = 1
Lone pairs = 3
FC = 6-2-(2*3) =-1
Another example:
Nitrogen (N)
It has 5 valence electrons so it is very happy with three bonds and one lone pair in the Lewis dot structures
Valence electrons of N = 5
No of bonds = 3
Lone pairs = 1
FC = 5-3-(2*1) =0
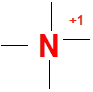
However if there are 4 bonds around N which we generally see in many ammonium compounds than it will acquire a formal positive charge
Valence electrons of N = 5
No of bonds = 4
Lone pairs = 0
FC = 5-4-(2*0) =+1
Yet another example:
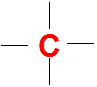
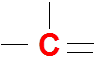
Carbon (C)
It has 4 valence electrons so it is very happy with four bonds and no lone pairs in the Lewis dot structures.
Valence electrons of C = 4
No of bonds = 4
Lone pairs = 0
FC = 4-4-(2*0) =0
The atoms discussed above are in the second period of the periodic table and hence cannot have more than 8 electrons in the outermost shell (no expanded octet due to lack of d orbitals).

Now, let’s take an element which can have an expanded octet.
Examples of expanded octet
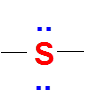
Sulfur/Sulphur (S)
It has 6 valence electrons. So, like oxygen it is also very happy with zero formal charge on it. However, unlike oxygen it has more different combinations to get a zero formal charge. One of the combinations is just like oxygen atom (two bonds and two lone pairs)
Valence electrons of S = 6
No of bonds = 2
Lone pairs = 2
FC = 6-2-(2*2) =0
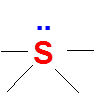
Second combination is four bonds and one lone pair .Here Sulfur has 10 electrons around it .(expanded octet and extra electrons are accommodated in the empty 3d orbitals of Sulfur).
Valence electrons of S = 6
No of bonds = 4
Lone pairs = 1
FC = 6-4-(2*1) = o
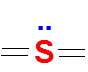
Third combination is 6 bonds and no lone pair . Here Sulfur has 12 electrons around it (expanded octet and extra electrons are accommodated in the empty 3d orbitals of sulfur)
Valence electrons of S = 6
No of bonds = 6
Lone pairs = 0
FC = 6-6-(2*0) =0
Formal Charge Flexibility in Phosphorus
Phosphorus, like nitrogen, has 5 valence electrons, so it is also most stable when it carries zero formal charge.
However, unlike nitrogen, phosphorus has more bonding possibilities that allow it to achieve this zero formal charge.
For instance, one common and stable arrangement is three bonds and one lone pair, just like nitrogen.
But in addition, phosphorus can also expand its octet (since it is in Period 3), allowing it to form structures with five bonds and no lone pairs, while still maintaining a formal charge of zero.
Therefore, when drawing Lewis structures involving phosphorus, it is essential to consider multiple valid arrangements, especially in molecules like PCl₅ or H₃PO₄.
Valence electrons of P = 5
No of bonds = 3
Lone pairs = 1
FC = 5-3-(2*1) =0
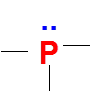
Second feasible combination to get zero formal charge is five bonds around P . Here phosphorus is with 10 electrons around it (expanded octet and extra electrons are accommodated in the empty 3d orbitals of Phosphorous)
Valence electrons of P = 5
No of bonds = 5
Lone pairs = 0
FC = 5-5-2*0=0
Now let us apply step 3 on our molecule
SO2
From step 2 skeleton of the molecule is
Completing the Octet of Oxygen with Minimal Formal Charge
Now that we’ve assigned the basic framework of the Lewis structure, let’s focus on completing the octet of the most electronegative element—oxygen.
As a general rule, when building a Lewis structure, we should always complete the octet of the more electronegative atoms first, and oxygen is no exception.
Based on what we’ve learned, oxygen is most stable when it forms two bonds and holds two lone pairs, which typically results in a formal charge of zero.
Therefore, to satisfy both the octet rule and maintain minimal formal charge, we can confidently assign a double bond and two lone pairs to each oxygen atom in the molecule.
This arrangement keeps the oxygen atoms stable while maintaining the overall charge balance of the molecule.
Let’s take nitrate ion as the next example.
In the nitrate ion – NO3−
From step 2 skeleton of the molecule is
Now that the basic skeleton of the molecule is in place, let’s move on to completing the octet of the most electronegative atom—oxygen—while also aiming for a minimal formal charge.
Since oxygen is usually found at the terminal position, and because it is highly electronegative, it prefers to maintain stability by forming a double bond and holding two lone pairs.
As a result, this arrangement not only satisfies the octet rule but also ensures that the formal charge on oxygen remains zero, making it a safe and reliable choice in most Lewis structures.
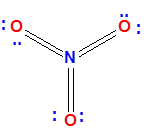
Why This Lewis Structure Is Incorrect for Nitrogen
At first glance, the structure might seem acceptable, but upon closer inspection, it violates a key rule: Nitrogen cannot have more than 8 electrons around it.
In the structure above, nitrogen is shown forming six covalent bonds, which translates to 6 × 2 = 12 electrons.
Clearly, this exceeds the octet limit for nitrogen, which belongs to the second period and does not have access to d-orbitals to expand its octet.
Therefore, we need to adjust the structure to make it chemically valid. Specifically, we should replace two of the double bonds between nitrogen and oxygen with single bonds.
As a consequence, the two oxygen atoms now forming single bonds with nitrogen require one additional lone pair each to complete their octets.
In the corrected version, you will observe that both of these oxygen atoms carry three lone pairs, which satisfies their octet and ensures formal charges are correctly assigned.

Step 4: Complete the Structure by Adding Remaining Valence Electrons as Lone Pairs
Now that the bonding framework is in place, the final step in drawing a correct Lewis structure is to place the remaining valence electrons—those not yet used in bonds—as lone pairs on the atoms, especially the central atom, if needed.
🔸 Let’s Apply This to an Example: SO₂ Molecule
-
Total valence electrons = 18
(This was calculated in Step 1 by adding 6 from sulfur and 6 from each of the two oxygen atoms.) -
Next, count the number of electrons used so far in the structure.
-
Every bond uses 2 electrons (1 pair).
-
Add up the total number of bonding electrons and lone pairs already placed.
-
-
Then, subtract this number from the total valence electrons (18 in this case).
-
The difference tells you how many electrons are still left to place.
-
-
Finally, assign the remaining electrons as lone pairs, starting with the outer atoms (usually oxygen), and place any extra pairs on the central atom (sulfur) if needed.
This step ensures that all atoms—especially oxygen and sulfur—achieve stable electron configurations, following the octet rule (or expanded octet for sulfur, since it’s in Period 3).
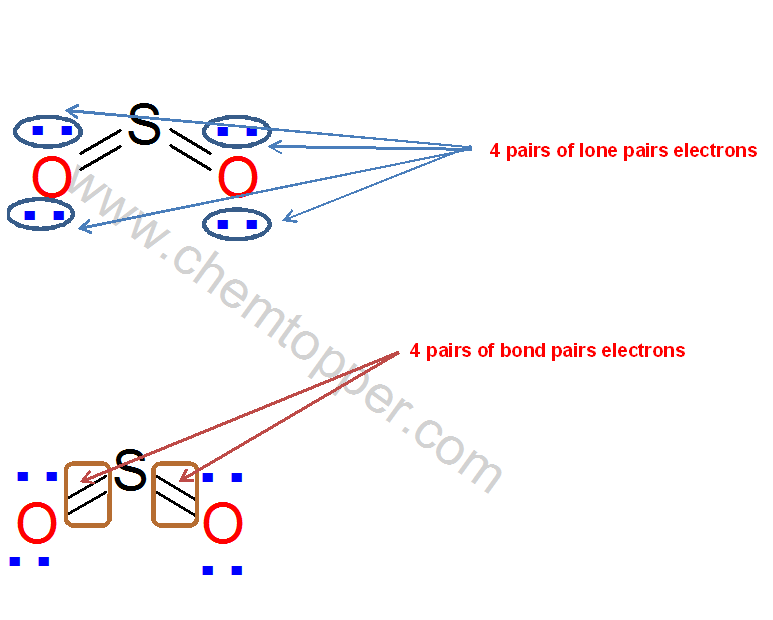
Number of electron used up to step 3 are
4 bond pairs and 4 lone pairs hence total is 4*2(Bond pair) +4*2 (lone pair) =16
No of electrons left unused = Total valence electrons – electrons used in Lewis dot structure
= 18-16 =2
These left electrons pair is put on the S atom
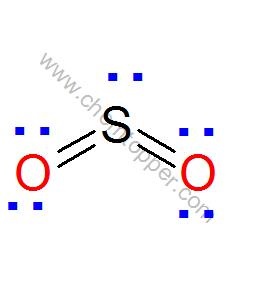
Now let us calculate the formal charge on each atom in the Lewis dot structure of SO2 molecule
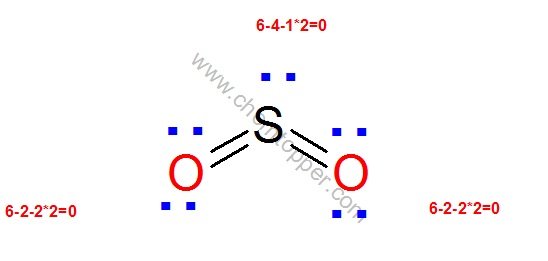
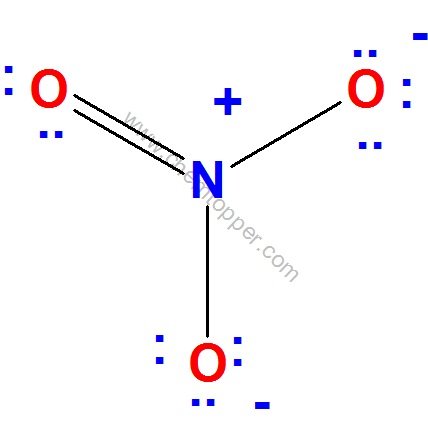
Now let us check for NO3– (nitrate ion)
Total valence electrons = 24
Electrons used are as 4 bond pairs and 8 lone pairs =4*2+8*2=24
Hence all 24 valence electrons are used up .
Let us calculate formal charge on each atom using the equation
FC = Valence electrons – No of bonds – 2*Lone pairs
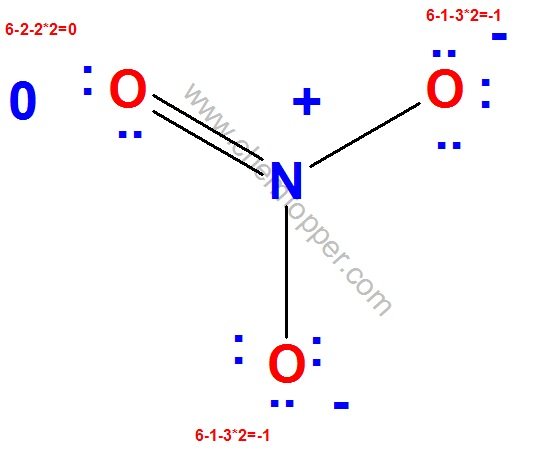
Final Lewis dot structure of NO3– (nitrate ion)
Mastering the 4 Key Steps to Draw a Correct Lewis Dot Structure
To accurately draw a Lewis dot structure, you need to follow a set of four essential steps. These steps help ensure your structure is both chemically valid and easy to interpret. Let’s break them down clearly:
Step 1: Count the Total Valence Electrons
First, determine the total number of valence electrons in the molecule or ion.
To do this, simply add the valence electrons from each atom involved.
For ions, don’t forget to add or subtract electrons depending on the charge (add for negative ions, subtract for positive).
Step 2: Choose the Central Atom and Build the Skeleton
Next, select the central atom—typically the least electronegative element (except hydrogen, which is always terminal).
Then, arrange the surrounding atoms to form the basic skeletal structure using single bonds.
Step 3: Complete the Octet of Outer Atoms with Minimum Formal Charges
Now, focus on completing the octet of the most electronegative atoms first (usually the terminal atoms) using lone pairs.
At the same time, aim to keep the formal charges as close to zero as possible.
Here’s how to calculate formal charge:
Formal Charge=Valence Electrons−Number of Bonds−2×Number of Lone Pairs\text{Formal Charge} = \text{Valence Electrons} – \text{Number of Bonds} – 2 \times \text{Number of Lone Pairs}
Or, alternatively:
Formal Charge=Group Number−Bond Pairs−2×Lone Pairs\text{Formal Charge} = \text{Group Number} – \text{Bond Pairs} – 2 \times \text{Lone Pairs}
Remember, structures with the lowest formal charges, especially on electronegative atoms, are generally more stable.
Step 4: Place Remaining Electrons as Lone Pairs on the Central Atom
Finally, if any valence electrons remain, place them as lone pairs on the central atom.
This step ensures all electrons are accounted for, and if the central atom is in the third period or beyond, it can expand its octet.
By mastering these four steps, you’ll be able to construct accurate Lewis dot structures for atoms, ions, molecules, and polyatomic ions with confidence.
Practice Examples on Lewis Dot Structure:
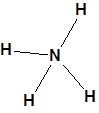
NH4+ (ammonium ion) Lewis Dot Structure
Step 1
Total valence electrons = 5(N) + 4*1 (4 H s)-1 (due to one positive charge) = 8
Step 2
Central atom is N because H can never be the central atom and N is more EN than H. (remember mentioned earlier also)
Skeleton of NH4+
Step 3 is already taken care of ,as N has 8 electrons around it and each H is with two electrons on it .
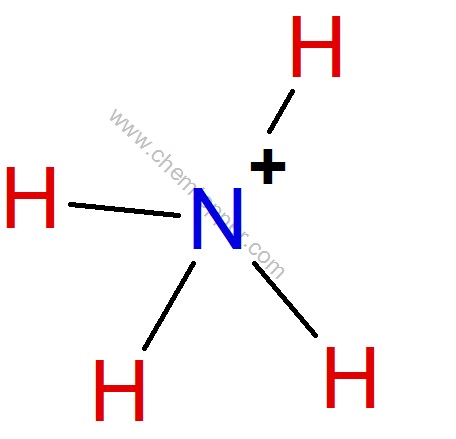
Step 4 :
Total electrons used are as 4 bond pairs = 4*2 = 8
Formal charge on N= Valence electrons – no of bonds – 2*Lone pairs
5-4-0 = +1
Formal charge on H = Valence electrons – no of bonds – 2*Lone pairs
= 1-1-0 = 0
Final correct Lewis dot structure of ammonium ion is:
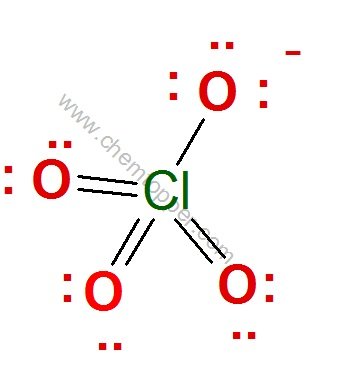
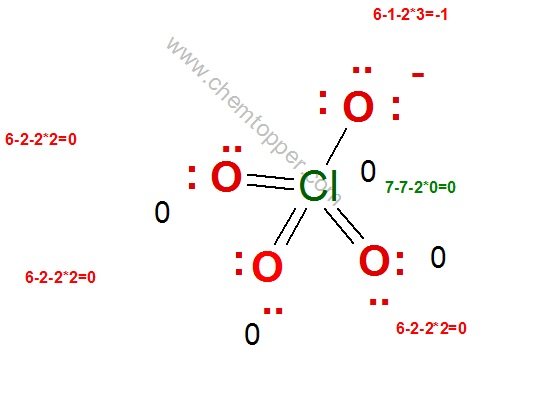
ClO4– ion (Perchlorate ion) Lewis dot structure
Step 1
Total valence electrons = 7(Cl) + 4*6 (4 O)+1 (due to one negative charge) = 32
Step 2
Central atom is Cl because O is more electronegative than Cl (check the periodic table)
Skeleton of ClO4– ion
Step 3
Complete the octet of oxygen with minimum formal charge .
Oxygen being terminal is very happy with a double bond and two lone pairs
Important Note on Chlorine and Its Bonding Limit
Always remember, chlorine can have a maximum of 7 valence electrons, which limits it to forming up to 7 bonds in a Lewis structure.
In the structure above, chlorine is shown forming 8 bonds, which exceeds its available valence electrons. As a result, chlorine ends up with a negative formal charge, making the structure less stable and chemically inaccurate.
To fix this, we can simply replace one of the double bonds between chlorine and oxygen with a single bond. Then, to complete the octet of the oxygen atom, we add an extra lone pair.
This small adjustment brings the formal charge on chlorine back to zero and maintains a valid octet for all atoms involved—resulting in a more stable and accurate Lewis structure.
(FC = Valence electrons – no of bonds – 2*Lone pairs)
Step 4:
Electrons used are as 7 bond pairs and 9 lone pairs = 7*2+9*2=32 electrons
Hence all valence electrons are used and no more electrons are left.
Final completed correct Lewis dot structure of perchlorate ion is