Electronegativity (EN): Definition, Trends, and Key Factors
What Is Electronegativity?
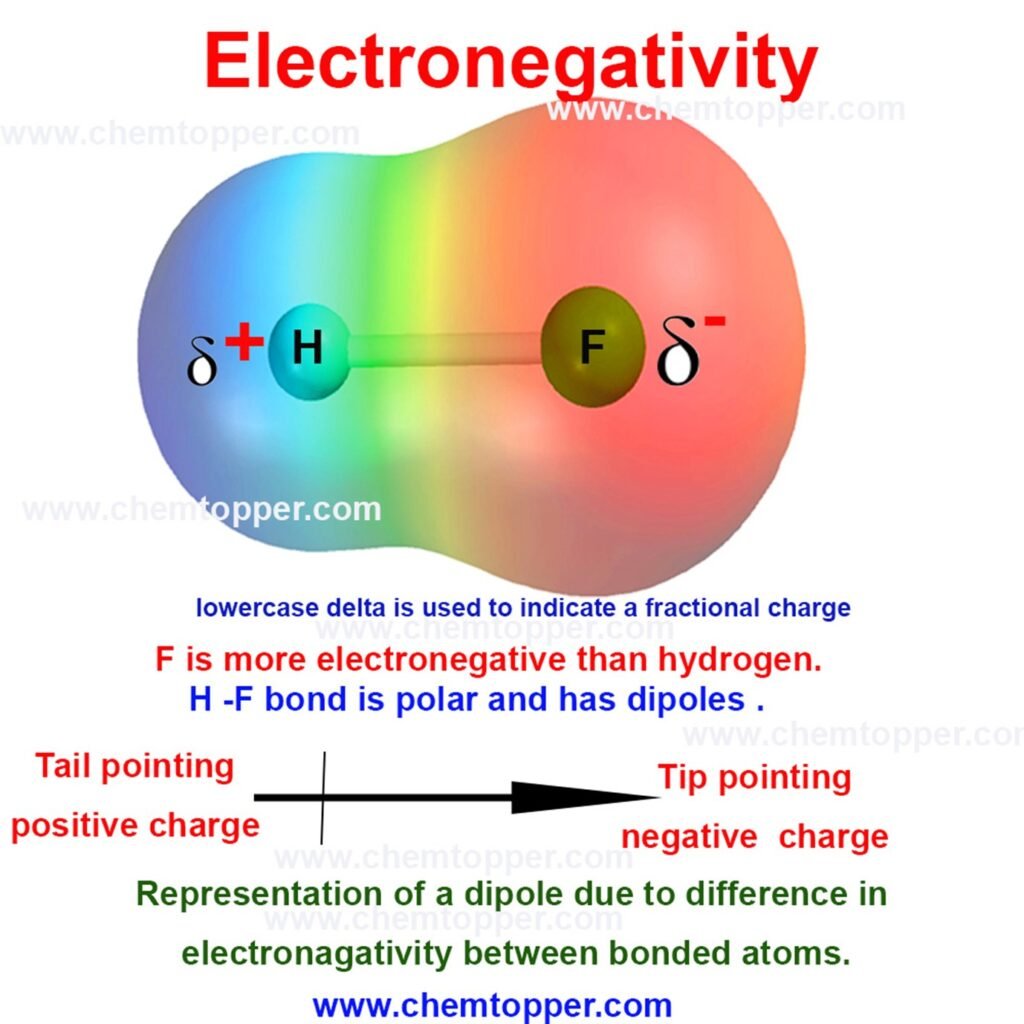
Electronegativity measures how strongly an atom pulls the shared pair of electrons in a covalent bond toward itself. Atoms that pull harder usually have a high effective nuclear charge (Zeff), small atomic size, and minimal electron shielding.
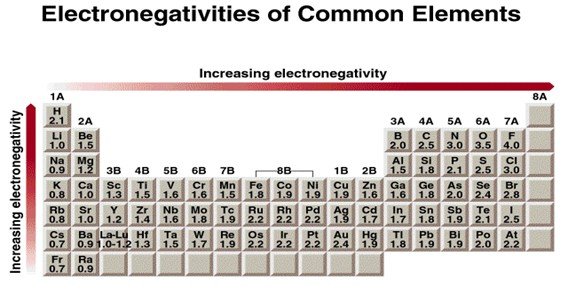
For example, fluorine ranks as the most electronegative element, while cesium shows the least tendency to attract bonding electrons.
How Electronegativity Changes in the Periodic Table
Factors Controlling The Electronegativity (EN)
Several factors influence how strongly an atom attracts shared electrons:
1. Effective Nuclear Charge (Z_eff)
Atoms with more protons pull electrons more strongly because they have a higher Zeff.
2. Atomic Radius
Smaller atoms bring bonding electrons closer to the nucleus, increasing EN.
3. Shielding Effect
When inner shells block the nuclear pull, atoms lose the ability to attract electrons effectively. Hence, more shielding leads to lower electronegativity.
Periodic Trends For Electronegativity (EN)
How Electronegativity Changes in the Periodic Table
Electronegativity follows predictable periodic trends. To understand how EN changes, you need to consider atomic structure, nuclear charge, and shielding effects.
Across a Period: Electronegativity Increases
As you move from left to right across a period, each atom gains more protons in its nucleus. At the same time, electrons continue to occupy the same energy level. Because of this:
- The effective nuclear charge increases
- The atomic radius decreases
- The nucleus pulls bonding electrons more strongly
Therefore, electronegativity increases steadily across the period.
Example:
Fluorine, located at the top-right corner (excluding noble gases), pulls bonding electrons most effectively, making it the most electronegative element.
Down a Group: Electronegativity Decreases
As you go down a group, atoms gain additional electron shells. As a result:
- The atomic size increases
- The valence electrons stay farther from the nucleus
- The shielding effect increases, which reduces Zeff
Because the nucleus cannot hold the bonding electrons as tightly, electronegativity decreases as you move down the group.
Example:
Cesium lies at the bottom of Group 1 and barely attracts bonding electrons, so it has the lowest electronegativity among stable elements.
Quick Summary For Electronegativity (EN).
- Electronegativity increases across a period
→ Because the nucleus gains protons and pulls electrons more tightly
→ Atomic size shrinks, and Zeff increases - Electronegativity decreases down a group
→ Because atoms add shells, expand in size, and increase shielding
→ The nucleus pulls less effectively on bonding electrons
Electronegativity values on Pauling Scale.