By Mehr Grewal
What is a particle? This is a question that scientists have been trying to answer for decades.
Fortunately, quantum mechanical breakthroughs have enabled us to gain a better understanding of what a particle is and how particles came to make up our world. More fundamental than the particles themselves, according to quantum mechanics, are their underlying quantum “fields.”
Quantum field theory defines a particle as an excited state, or quanta, of its underlying quantum field.
To understand what this means, we must first examine the history of quantum field theory, which really begins hundreds of years ago with Isaac Newton and his law of universal gravitation. It was puzzling to him how objects that had never come in contact could exert a force on each other. Newton reasoned that there had to be something mediating the interactions between distant objects.
Later, in 1845, Michael Faraday formulated our modern definition of a “field.” He speculated that instead of considering the gravitational force as “action at a distance,” it should instead be considered invisible “lines of force” that connected distant objects.
Later, physicists came to realize that light is “quantized;” it cannot be absorbed or emitted in any arbitrary amount and instead occurs in “packets” of energy initially called photons. It is important to note that we usually hear the term “quanta” as synonymous with photons, but photons are better defined as the quanta of light. In truth, all force is quantized, a central point of the quantum field theory.
Until the advent of quantum electrodynamics, particles and quantum fields were treated as 2, separate, noninteracting “ingredients.” In the 1920s, however, scientists began contemplating the interaction between light and electrons as a possible source of the electromagnetic force. This work was supported by the Standard Model, which led to its wide acceptance.
At this time, scientists were also trying to explain a process called spontaneous emission. Spontaneous emission occurs when a quantum mechanical system releases energy in the form of a photon to “spontaneously decay” to a more stable ground state.
Spontaneous emission is the source of most of the light we see around us. The equation for spontaneous emission is as below:
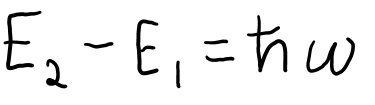
E2 is the energy of the system in the excited state and E1 is the energy of the system at ground state. H-bar is the Reduced Planck Constant. H-bar is defined as h/2pi , and it is used when we wish to talk about a particle in terms of revolutions or rotations. W is the angular frequency, which is measured in rotations (radians) per second.
It is crucial to note that:
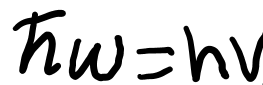
and both define a photon in terms of its energy.
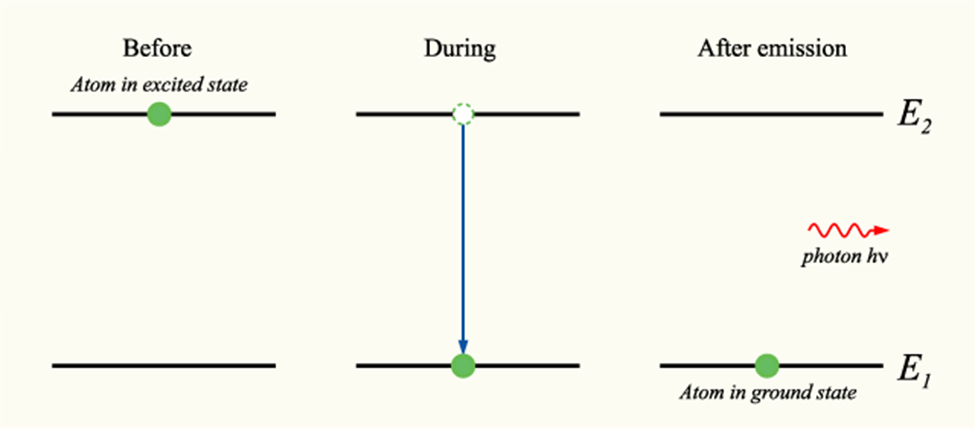
It was deduced by physicists that even in a vacuum, an oscillating electromagnetic field is present that results in spontaneous emission of particles.
After this discovery, a team of scientists led by the leading theoretical physicists at the time including Werner Heisenberg, Pascual Jordan, and Enrico Fermi realized that each fundamental particle corresponded to a respective quantum field that, when energized, would result in the production of these particles.
As predicted by the Standard Model, there are 24 fundamental quantum fields; however, the Universe itself can be described as one quantum field that explains all the particles that exist.
Admittedly, quantum field theory is an ambiguous area that stands at the frontiers of physics. However, as physics progresses toward a single Unified Theory of Everything that explains the entire Universe, the veil of mystery surrounding quantum mechanics will finally begin to lift.