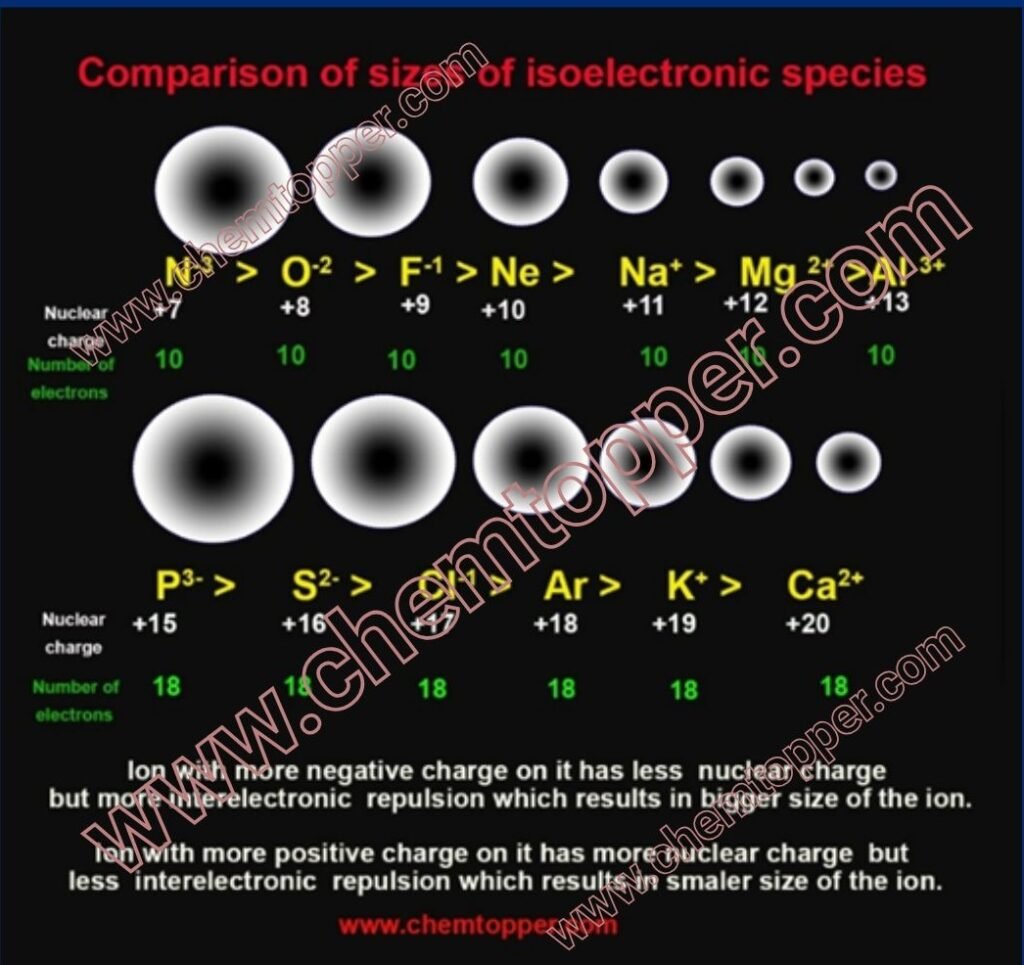
Isoelectronic Species and Ionic Size Comparison
When comparing ions, we often come across isoelectronic species—ions or atoms that have the same number of electrons, but different nuclear charges. Even though they share the same electron configuration, their sizes vary significantly due to differences in the number of protons pulling on those electrons.
What Are Isoelectronic Species?
Isoelectronic species are atoms or ions that have identical electron configurations. Even though they “look alike” in terms of electron distribution, they behave differently because their nuclei differ in the number of protons, which changes how tightly they hold their electrons.
For example:
- O²⁻, F⁻, Na⁺, Mg²⁺, and Al³⁺ all have 10 electrons, and therefore the same electron configuration:
1s22s22p6
Then Why Do Sizes of Isoelectronic Species Differ?
Although the electron count is the same, the number of protons in the nucleus increases as you move from left to right in the isoelectronic series:
O²⁻ → 8 protons and 10 electrons
F⁻ → 9 protons and 10 electrons
Na⁺ → 11 protons and 10 electrons
Mg²⁺ → 12 protons and 10 electrons
Al³⁺ → 13 protons and 10 electrons
As a result, the effective nuclear charge (Zeff) increases across the series, pulling electrons closer to the nucleus and making the ion smaller.
Cations with greater positive charges (e.g., Al³⁺ vs. Mg²⁺) exhibit even smaller radii. The loss of additional electrons intensifies the effective nuclear charge, drawing the electron cloud nearer to the nucleus. For instance, Al³⁺ has a smaller radius than Mg²⁺ due to the higher positive charge exerting a stronger pull on the remaining electrons.
Na⁺ (11 protons) < Mg²⁺ (12 protons) < Al³⁺ (13 protons)
Anions are larger due to lower nuclear charge and increased electron-electron repulsion.More negative charge on the ion means more bigger size due to more expansion to minimize interelectron repulsion.
N³⁻ > O²⁻ > F⁻
Order of Size Of Isoelectronic Species : Largest to Smallest
Although these species have the same number of electrons, the atomic/ionic radius decreases as the nuclear charge increases.
Why Isoelectronic species have different Sizes?
Because more protons pull the electrons closer to the nucleus, increasing effective nuclear charge (Zeff) and shrinking the radius.
O²⁻>F⁻>Na⁺>Mg²⁺>Al³⁺
- O²⁻ has the fewest protons for the number of electrons → weakest pull, largest radius
- Al³⁺ has the most protons for the same number of electrons → strongest pull, smallest radius
Key Takeaways For Comparison Of Sizes of Isoelectronic Species
Isoelectronic species have equal electrons, but different sizes.
More protons = stronger pull on electrons = smaller size.
This trend is useful when comparing ions in the same period or within ionic compounds.
Why Are Isoelectronic Species Important?
- In ionic compounds, the size of an ion affects lattice energy and bond strength.
- In periodic trends, understanding size variation in isoelectronic ions helps explain why cations are smaller and anions are larger than their neutral atoms.
- In chemical reactivity, ions with the same configuration may respond differently in reactions due to differences in size and nuclear charge.