Basic Nature of Oxides – Periodic Trends Made Simple
What Are Basic Oxides?
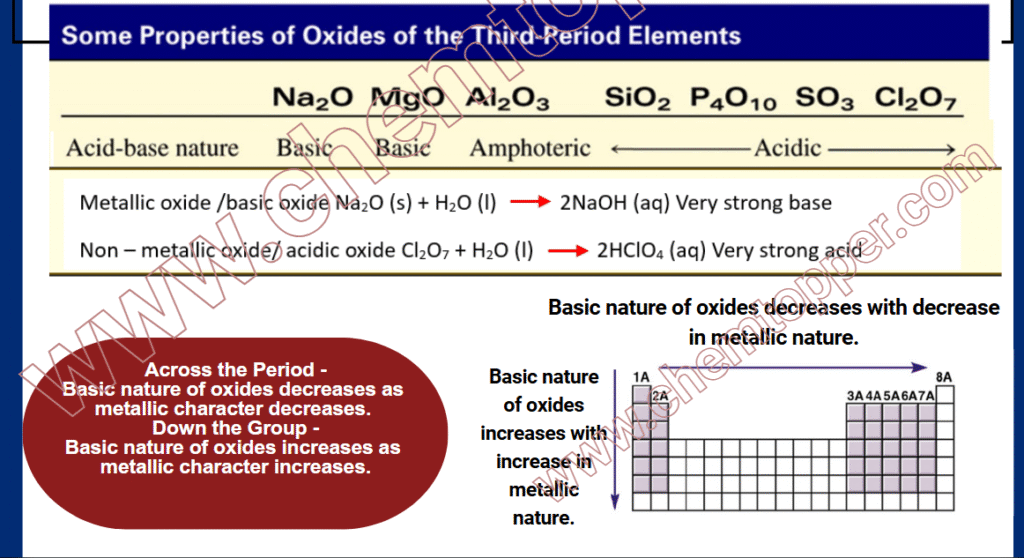
Basic oxides form when metallic elements react with oxygen. When you add these oxides to water, they produce metal hydroxides, which act as strong bases.
For example:
- Na₂O + H₂O → 2NaOH (aq)
- MgO + H₂O → Mg(OH)₂ (s)
So, elements with strong metallic character tend to form basic oxides.
Key Factor: Metallic Character
The more metallic an element is, the stronger its basic oxide becomes.
Some classic basic oxides include:
- K₂O, CaO, BaO, Al₂O₃
Metals like potassium, calcium, and barium form oxides that show high basic strength. Therefore, you can easily link metallic character to the basic nature of oxides.
Periodic Trends in Basic Nature of Oxides
Across a Period (Left to Right): Basic Nature Decreases
As you move from left to right in a period:
- The metallic character decreases
- Atoms hold electrons more tightly
- Ionization energy increases
- Elements shift from metallic to non-metallic
- Hence, their oxides become less basic and more acidic or amphoteric
For example:
- Na₂O and MgO are strong basic oxides
- Al₂O₃ is amphoteric (shows both acidic and basic behavior)
- SiO₂, P₄O₁₀, SO₃, and Cl₂O₇ are acidic oxides
Thus, the basic nature of oxides decreases as you go across a period.
Down a Group (Top to Bottom): Basic Nature Increases
Now, let’s go down a group:
- Atomic size increases
- Valence electrons stay farther from the nucleus
- Metallic character increases
- Electron loss becomes easier
- As a result, elements form more strongly basic oxides
Most alkali metals (Group 1) and alkaline earth metals (Group 2) form oxides that are highly basic in nature.
For example, Cs₂O and BaO form very strong bases in water.
Properties of Third-Period Oxides – A Quick Comparison for Basic Nature of Oxides
Basic Nature of Oxides Periodic Trends
Clearly, as metallic character decreases across the period, the acidic nature of oxides increases.
Final Takeaway for Basic Nature of Oxides Periodic Trends:
Basic nature of oxides decreases across a period, as elements become less metallic
- Basic nature increases down a group, as metallic character strengthens
- Alkali and alkaline earth metals form the strongest basic oxides
- Halogens and other nonmetals form acidic oxides with water