Electron Affinity (EA) – Definition, Trends, and Exceptions
What Is Electron Affinity?
Electron affinity (EA) refers to the energy change that occurs when a gaseous atom gains an electron to form a negative ion (anion):
X(g) + e⁻ → X⁻(g)
When an atom attracts an incoming electron, it forms a new electrostatic interaction between the nucleus and the added electron. This attraction releases energy, which we call electron affinity (EA) or electron gain enthalpy (∆He.g).
Key Points About Electron Affinity(EA)
- EA is usually an exothermic process (energy released), so the value is negative.
- EA is measured in kilojoules per mole (kJ/mol).
- EA is also called electron gain enthalpy (∆He.g).
- A more negative EA means the atom strongly attracts an electron.
- A less negative EA means the atom has a weaker tendency to gain an electron.
- A positive EA indicates no tendency to gain an electron—as seen in noble gases.
- A nearly zero EA suggests a very low tendency to accept an electron.
Example Values:
- F(g) + e⁻ → F⁻(g) ∆H = –328 kJ/mol
- O(g) + e⁻ → O⁻(g) ∆H = –141 kJ/mol
Factors Affecting Electron Affinity
1. Atomic Size
- Smaller atoms: Smaller atoms hold valence electrons closer to the nucleus. As a result, they attract additional electrons more effectively and release more energy. So, smaller atoms show higher (more negative) EA.
- In contrast, larger atoms have valence shells farther from the nucleus. The incoming electron experiences weaker attraction, so the atom releases less energy. Therefore, larger atoms show lower EA.
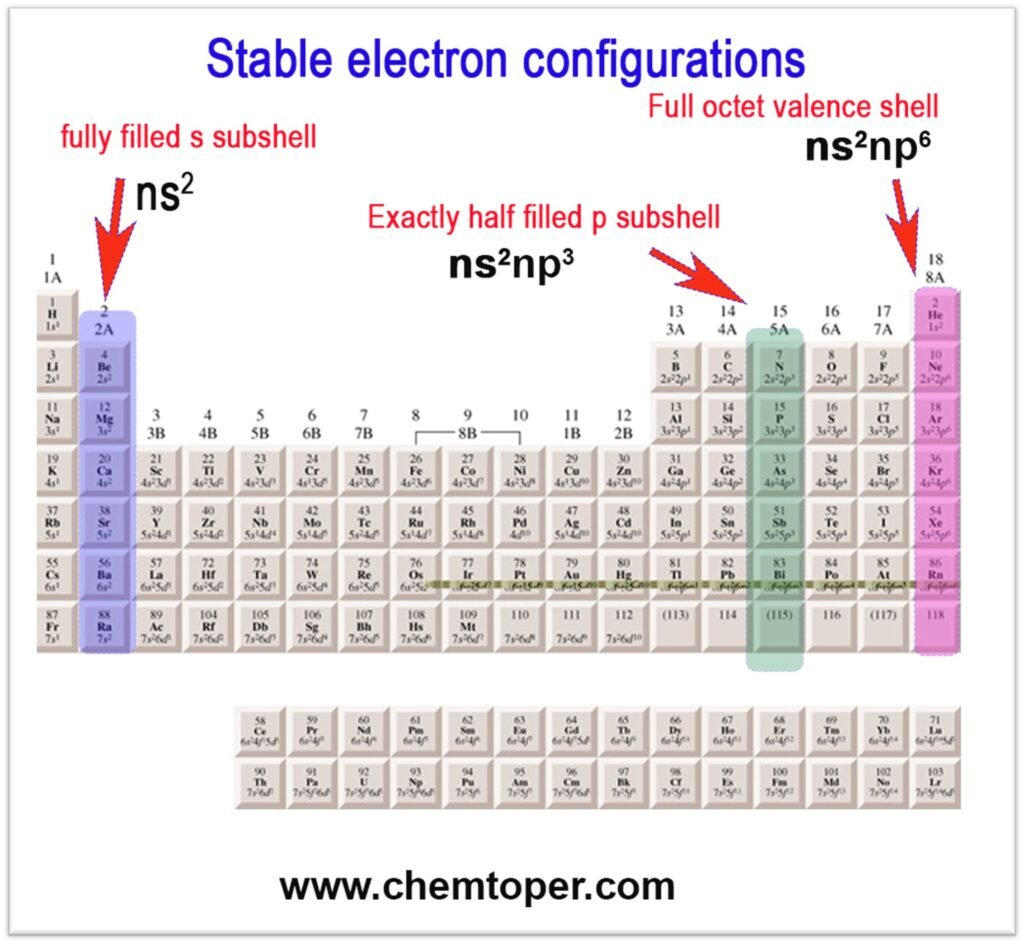
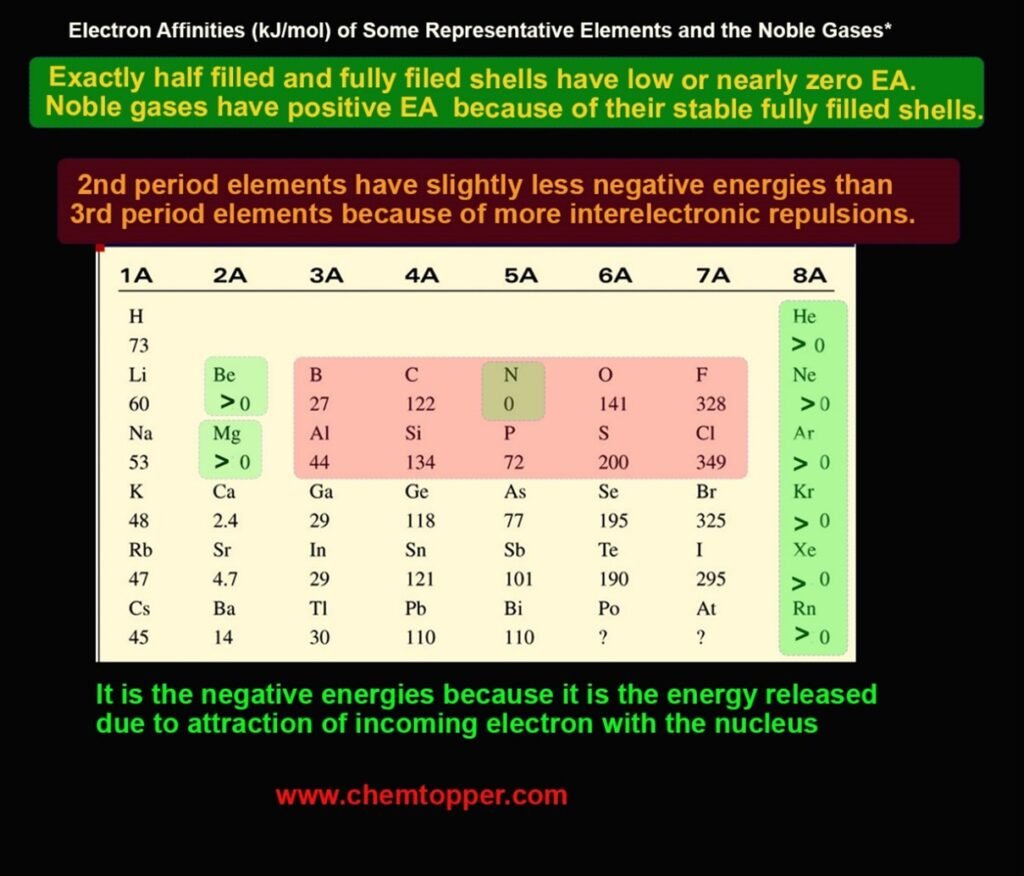
2. Stability of Electron Configuration
When an atom already has a stable configuration, it doesn’t want to gain another electron. For example, elements in the beryllium family (Group IIA) with a filled s-subshell and those in the nitrogen family (Group VA) with a half-filled p-subshell resist gaining electrons. Consequently, these elements show lower electron affinity than their neighbors.
3. Inter-electronic Repulsion
Atoms in Period 2 (like F, O, N) have small atomic sizes. When they accept an electron, the added electron faces strong repulsion from existing electrons in their compact orbitals. This reduces the energy released and results in lower-than-expected EA values.
For instance, even though fluorine is more electronegative than chlorine, it shows less negative EA due to stronger inter-electron repulsions.
Important Note About Second Electron Gain Enthalpies :
- Second electron gain enthalpies are always positive, as the electron is being added to an already negative ion, increasing repulsion.
O⁻(g) + e⁻ → O²⁻(g) ∆H=+780kJ/mol
- The first EA is negative (energy released).
- The second EA is positive (energy required to overcome repulsion).
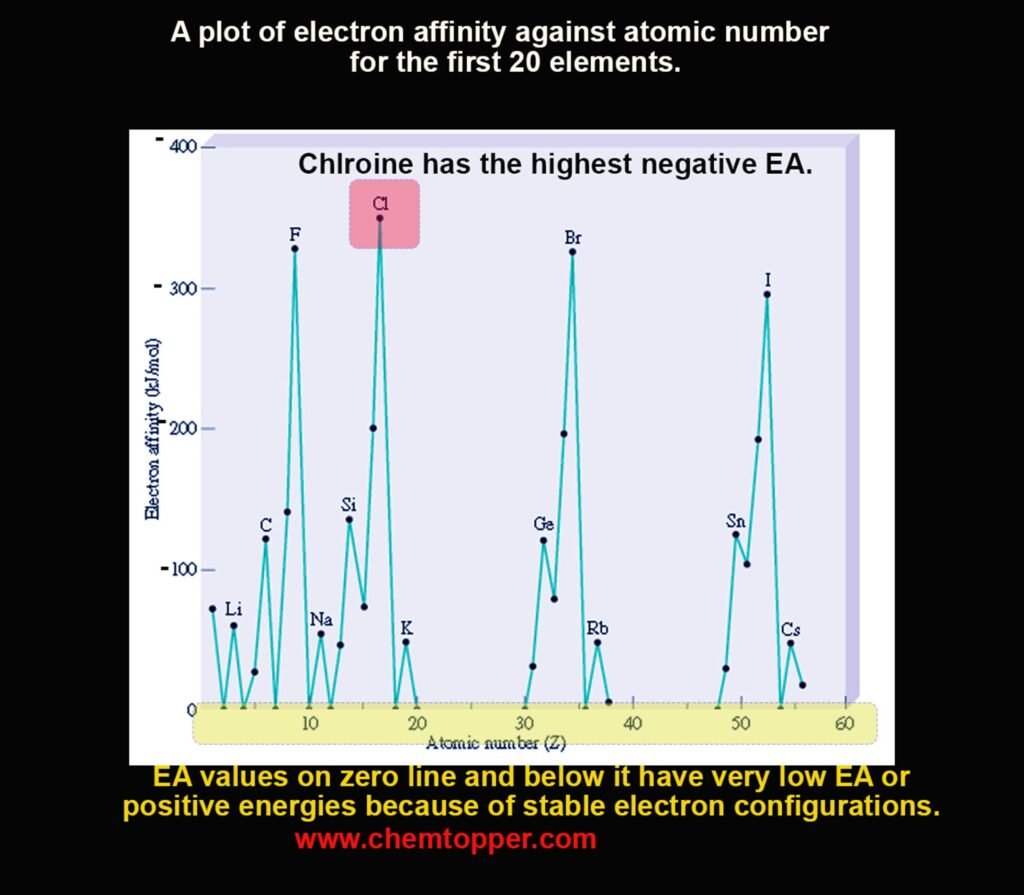
Periodic Trends in Electron Affinity
Down a Group: EA Becomes Less Negative
- As atomic size increases, the distance between the nucleus and valence shell increases.
- This weakens nuclear attraction, so less energy is released when an atom gains an electron.
Exception: Second Period Elements
- Elements like O, N, F have smaller EA than expected due to high inter-electronic repulsion in their compact orbitals.
Observed trend:
EA F < Cl, EA O < S, EA N < P
Chlorine has the highest (most negative) electron affinity in the periodic table.
Across a Period: EA Becomes More Negative
- Atomic size decreases → electrons are added closer to the nucleus → stronger attraction → more energy released
- Electron affinity increases (becomes more negative) across a period from left to right.
Exceptions Across a Period
Some groups show unexpected dips in EA due to electronic stability:
- Group IIA (ns²): Full s-subshell → low EA
- Group VA (ns²np³): Half-filled p-subshell → low EA
- Group 18 (noble gases): Full shells → positive EA (adding an electron requires energy)
Final Takeaway For Periodic Trends In Electron Affinity
- EA becomes more negative across a period and less negative down a group
- Atoms with stable configurations or high inter-electron repulsion resist gaining electrons
- Second EA values are always positive due to repulsion in anions
- The most negative EA is found in Group 17 (halogens)—especially chlorine