Periodic Trends In Ionization Energy: Definition, Trends & Exceptions
What Is Ionization Energy?
Ionization energy (IE) is the minimum amount of energy required to remove one mole of electrons from one mole of gaseous atoms in their ground state, resulting in 1+ cations.
It’s an endothermic process, meaning energy must be supplied to overcome the electrostatic attraction between the negatively charged electron and the positively charged nucleus.

IE of Potassium (K)=+419kJ/mol (for example)
Key Points to Remember about Ionization Energy(IE)
- IE is always positive because energy is absorbed.
- It is measured in kilojoules per mole (kJ/mol).
- Energy is used to break the attractive force between the nucleus and the electron.
- First Ionization Energy (IE₁)
- The first ionization energy refers to the energy needed to remove the first electron from a neutral atom: X (g)→X⁺ (g)+e⁻
Factors Affecting Periodic Trends In Ionization Energy (IE)
Ionization energy is mainly depends on Two Factors:
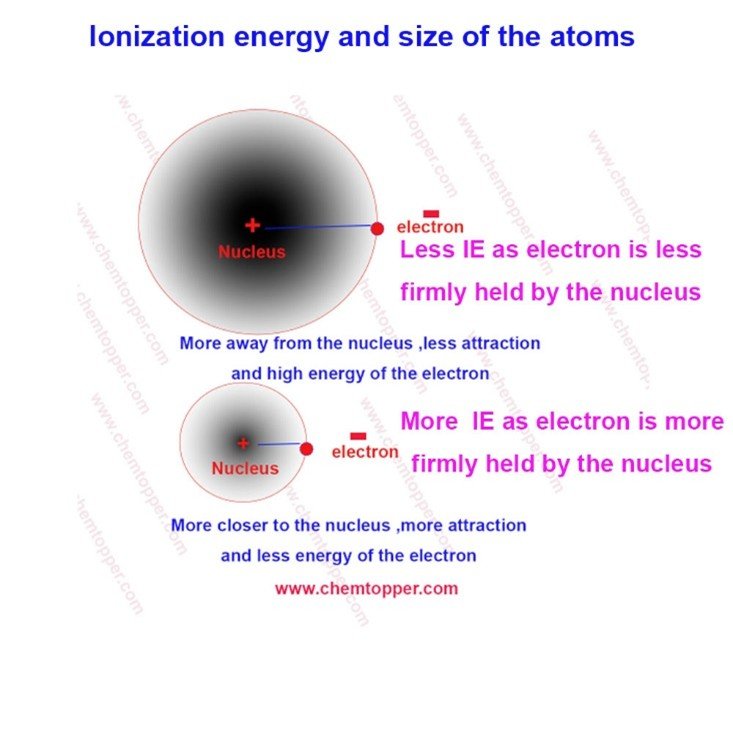
1. Atomic Size
- Smaller atoms: Electrons are closer to the nucleus → stronger attraction → higher IE
- Larger atoms: Electrons are farther from the nucleus → weaker attraction → lower IE
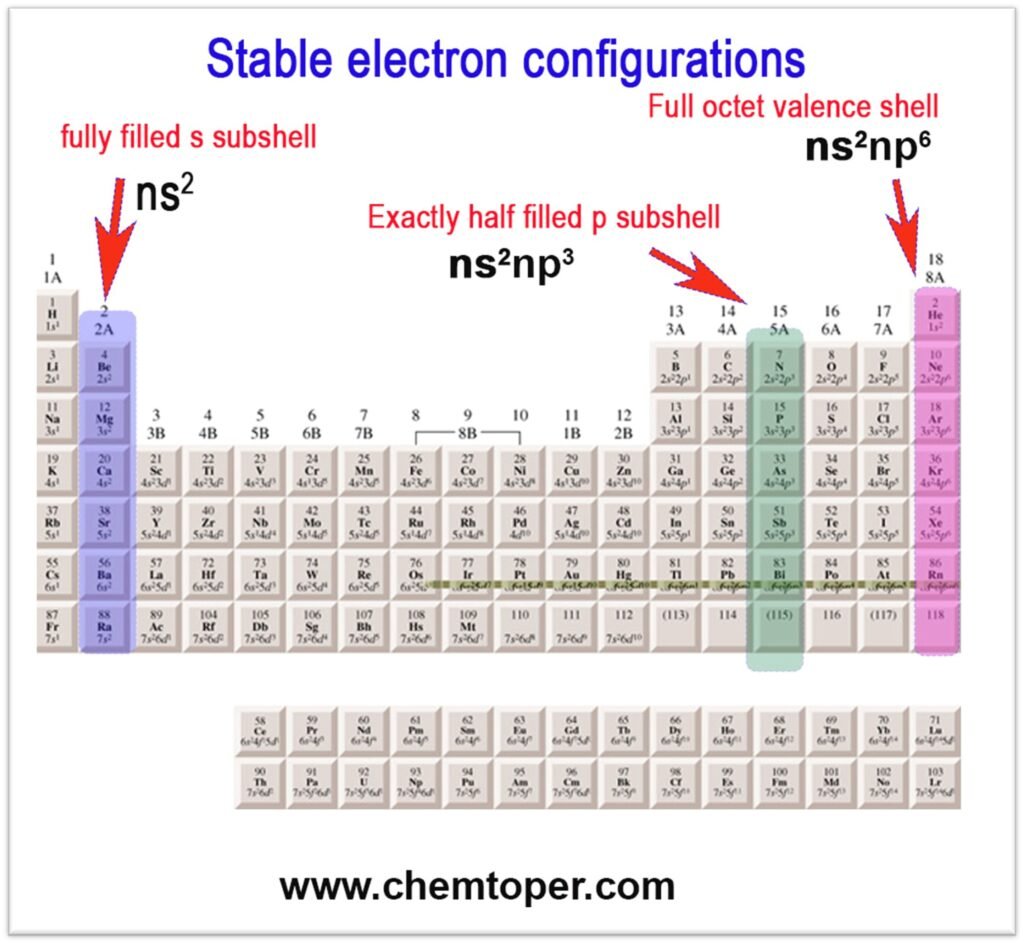
2. Stability of Electron Configuration
Atoms with stable configurations (like full or half-filled subshells) resist losing electrons, so their ionization energy is relatively higher.
- Beryllium group (Group IIA) with full s-orbital (ns²)
- Nitrogen group (Group VA) with half-filled p-orbitals (ns²np³)
These atoms have slightly higher IE than expected.
Keep in mind that exactly half-filled, fully filled, and noble gas configurations provide extra stability. For example, the nitrogen family has an exactly half-filled p-subshell, the alkaline earth metals (Group IIA) have a fully filled s-subshell, and the noble gases feature completely filled outer shells, making them the most stable of all.
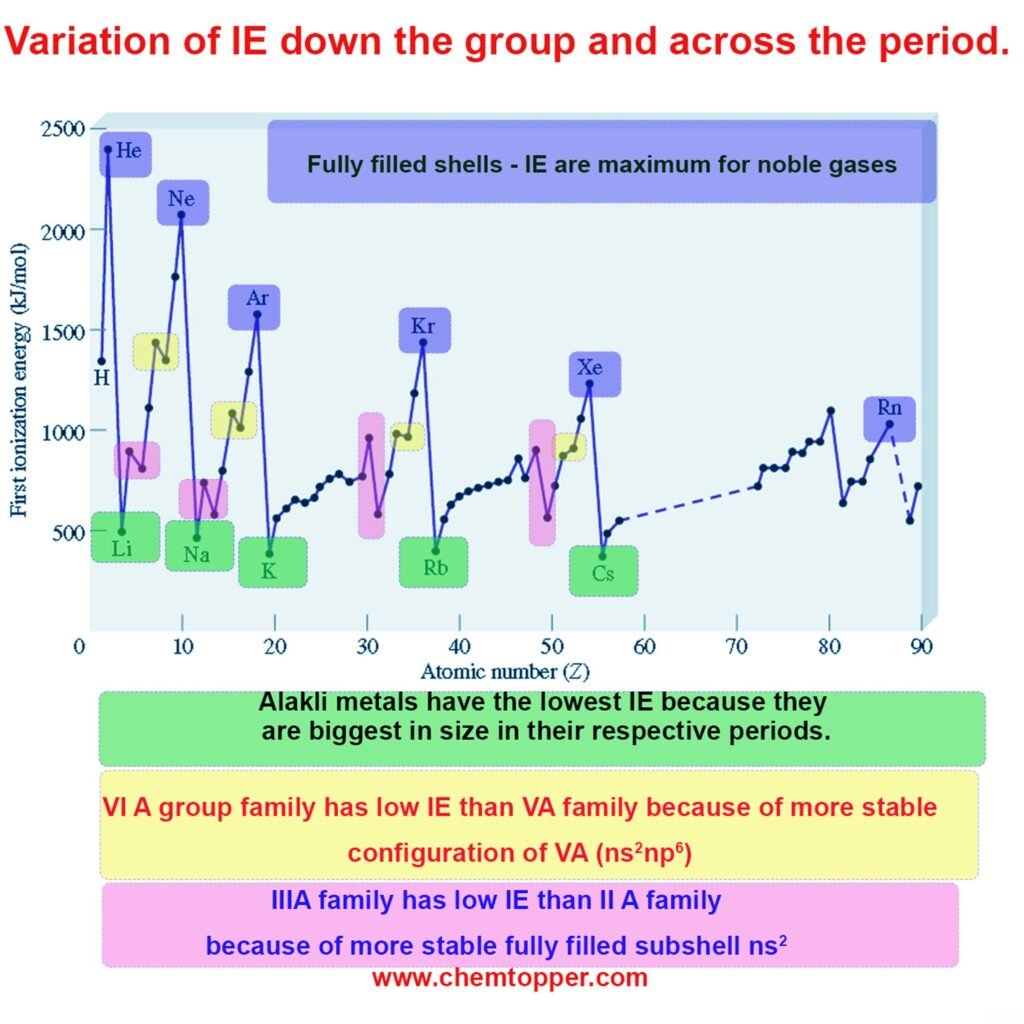
Periodic Trends in Ionization Energy
Down a Group
As atomic size increases, ionization energy decreases. In larger atoms, the outer electrons move farther from the nucleus, which weakens the electrostatic attraction. Therefore, the atom requires less energy to remove an electron.
Across a period
As atomic size decreases, ionization energy increases. The nucleus holds the electrons more closely, which strengthens the attractive force. Consequently, removing an electron becomes more difficult, and the atom requires more energy to do so.
Exceptional behavior across the period
Exceptions to the trend across a period occur due to extra stability associated with specific electron configurations. These breaks in the regular pattern are observed in cases such as:
- Fully filled subshells like ns² in Group IIA ( Alkaline earth metals)
- Exactly half-filled p-orbitals like ns²np³ in Group VA (Nitrogen family)
These stable arrangements resist electron removal, leading to higher-than-expected ionization energies.
Why Are Ionization Energy Exceptions Not Observed Down a Group?
Since all elements in a group have the same general valence shell configuration, they maintain similar stability in their outermost electrons.
Therefore, we don’t observe exceptional behavior down a group because elements have similar valence configurations and don’t gain extra stability from half-filled or fully filled orbitals.