Metallic Character Of Elements: Trends Across the Periodic Table
What Does Metallic Character Of Elements Mean?
Concept
Metallic character Of Elements refers to an atom’s tendency to lose electrons and form positive ions.
For example:
Mg → Mg²⁺ + 2 electrons
Na → Na⁺ + 1 electron
Factors Controlling Periodic Trends For Metallic Character Of Elements
- Atomic size
- Ionization energy
- Atoms with larger size and lower ionization energy lose electrons more easily, making them more metallic.
- In contrast, smaller atoms with high ionization energy tend to hold onto electrons more tightly, showing less metallic character.
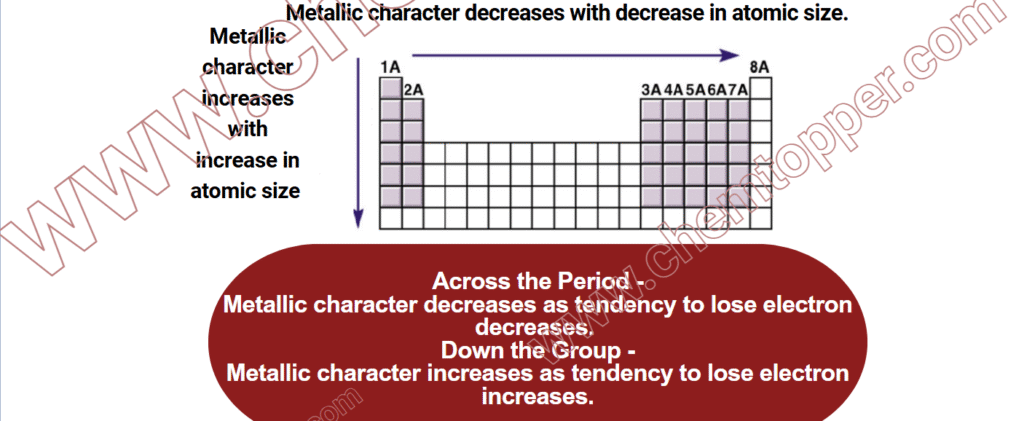
Periodic Trends In Metallic Character Of Elements :Across a Period
- As you move from left to right in a period, atomic size decreases.
- The number of protons increases, which increases the effective nuclear charge (Z_eff).
- This stronger nuclear attraction pulls electrons more tightly, making it harder for atoms to lose electrons.
- As a result, ionization energy increases, and metallic character decreases.
Observation :
- Nonmetals like F, O, Cl, and N, located on the far right of the periodic table, are the least metallic.
- Alkali metals (Group 1) show the highest metallic character in each period.
- Alkaline earth metals (Group 2) are also reactive but slightly less than alkali metals.
Down a Group (Top to Bottom): Metallic Character Increases
- Moving down a group, atomic size increases as atoms gain more electron shells.
- Even though protons are added, the increase in distance and shielding effect reduces Z_eff.
- As a result, atoms lose electrons more easily, and their metallic character increases.
- Ionization energy decreases down the group, supporting easier electron loss.
Observation:
- Elements like cesium (Cs), barium (Ba), rubidium (Rb), and strontium (Sr)—found in the bottom-left corner of the periodic table—show the strongest metallic behavior.
Metallic Character across the Period and down the group
Summary For Periodic Trends For Metallic Character Of Elements
Metals tend to lose electrons, so low ionization energy, large size, and weak Z_eff favor high metallic character.
Hence, elements become less metallic across a period and more metallic down a group.