What Is Ionization Energy & Successive Ionization Energies?
Ionization energy (IE) is a measure of how strongly an atom holds onto its electrons.
The higher the ionization energy, the harder it is to remove an electron from the atom. It reflects how tightly the nucleus attracts the electrons in a given energy level.
What Are Successive Ionization Energies?
In atoms with more than one electron, we can remove electrons one at a time, and each removal requires a specific amount of energy:
- First Ionization Energy (IE₁): Energy needed to remove the first electron X(g)+energy→X⁺(g)+e− IE1
- Second Ionization Energy (IE₂): Energy needed to remove the second electron X⁺(g)+energy→X²⁺(g)+e− IE2
- Third Ionization Energy (IE₃): Energy needed to remove the third electron X²⁺(g)+energy→X³⁺(g)+e− IE3
And so on…
Why Does Successive Ionization Energy Increase with Each Step?
After the first electron is removed, the atom becomes a positively charged ion. This reduces electron-electron repulsion and increases the effective nuclear attraction on the remaining electrons.
As a result, each successive electron is harder to remove, so each successive ionization energy is higher than the previous one.
Successive Ionization energies always increase in the following order:
IE1 < IE2 < IE3 < And so on…
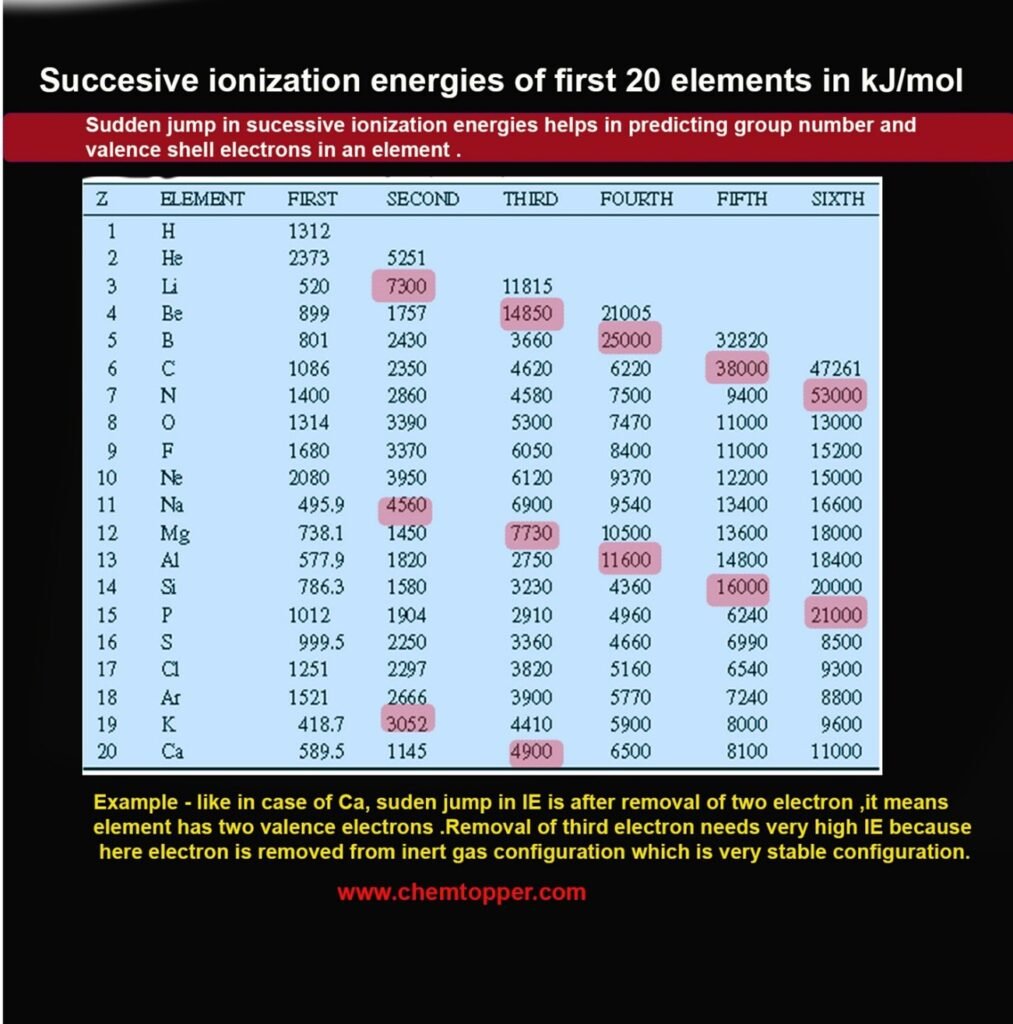
Sudden Jumps in Successive Ionization Energy
One of the most important patterns in successive ionization energies is the sudden, sharp increase that occurs after all valence electrons have been removed.
This dramatic jump signals that the next electron must be removed from a fully filled inner shell, which is much closer to the nucleus and much more tightly held.
Examples:
Sodium (Na):
Electron configuration: 1s² 2s² 2p⁶ 3s¹
- IE₁ = 495.9 kJ/mol (removing the 3s¹ valence electron)
- IE₂ = 4560 kJ/mol (removing from a stable 2p⁶ core) → Huge jump
Conclusion: Sodium has 1 valence electron → Group 1 (1A)
Magnesium (Mg):
Electron configuration: 1s² 2s² 2p⁶ 3s²
- IE₁ = 738.1 kJ/mol
- IE₂ = 1450 kJ/mol
- IE₃ = 7730 kJ/mol → Big jump after removing 2 valence electrons
Conclusion: Magnesium has 2 valence electrons → Group 2 (2A)
Using IE Jumps to Identify Group Number
- Large jump after IE₃ → 2 valence electrons → Group 2 (2A)
- Large jump after IE₅ → 4 valence electrons → Group 14 (4A)
- Large jump after IE₇ → 6 valence electrons → Group 16 (6A)
- Large jump after IE₈ → 7 valence electrons → Group 17 (7A)
Importance of Periodic Trends Of Successive ionization energies
- Successive ionization energies always increase, but the magnitude of the jump reveals the number of valence electrons.
- The first sharp increase in IE indicates when you’re entering the core electron region.
- This pattern is extremely useful in determining an element’s group number and understanding its reactivity.