Today, I will show you how to draw the Lewis structures of compounds. Lewis structures are a way to represent the ways electrons are arranged around an atom, transferred, or shared. Let’s start off by drawing the Lewis structures of individual atoms. To draw the Lewis structure of an atom, you should be familiar with the orbital box diagram, a way to represent electron configurations as “up” and “down” spins in an orbital. Let’s start off with chlorine as an example. Because Lewis structures only represent valence electrons, we only need to write the noble-gas configurations.
The electron configuration of chlorine is:
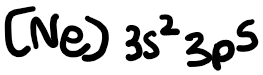
Next, we need to draw the orbital box diagram. We know that the s subshell has one orbital, so we only draw one box to represent 3s. The p subshell has 3 orbitals since the subshell can fit 6 electrons and 2 electrons maximum can be present in each orbital.
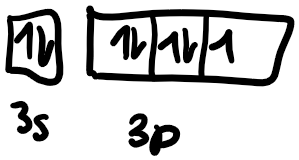
Following the Aufbau principle, electrons fill the lowest available energy levels first, so we start with the 3s orbital. Chlorine has 7 valence electrons total, and has a full 3s orbital, so we draw 2 electrons, one with an “up” spin, the other with a “down” spin. As a convention in chemistry, we always draw the electron with the “up” spin first. Hund’s rule states that for a group of orbitals in the same subshell, each orbital must be singly occupied by an electron before sharing an orbital. After we’ve filled the 3s subshell in chlorine, we have the 3p subshell to fill with 7-2=5 electrons. So we first fill the px orbital with a single orbital, then the py orbital, then the pz orbital. We need to fit in 2 more electrons, so we draw one with a “down” spin in the px orbital and then another “down” spin electron in the py orbital. After we’ve drawn the orbital box diagram, we can now get started on the Lewis structure. We start off by drawing the symbol in the center, then draw 2 electrons at the top to represent the 3s electrons. Then we draw one electron each on the remaining 3 sides and then pair the remaining last two.
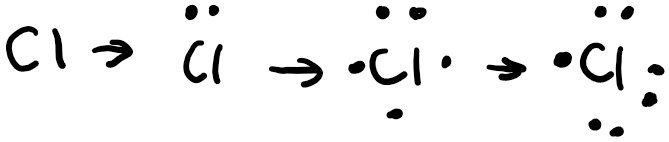
As you can see, chlorine has an unpaired electron which makes it very reactive. To achieve stability, it would react with another element, either by sharing electrons in a covalent bond or receiving electrons in an ionic bond.
Let’s draw the Lewis structure of hydrogen chloride, or hydrochloric acid in an aqueous solution.
1st step: Select the central atom which is least electronegative. Hydrogen and fluorine can never be the central atom, nor can they make a double bond. In this case, we don’t have a central atom since there are only 2 atoms.
2nd step: Count the total electrons! This is one of the most important steps and make sure that you always know how many electrons you have and how many you have used. Hydrogen has one valence electron, while chlorine has 7, so the total number of electrons should be 8.
3rd step: Draw the “skeleton,” or outline of the molecule. In this case, we know it will have a linear shape, with a single bond since hydrogen only has one valence electron (and can therefore make only a single bond.
4th step: Draw the lone pairs and check the “formal charge” on each atom. The formal charge is the number of valence electrons you have minus the number of valence electrons that have been used either as lone pairs or those of that atom in bonding. When drawing a Lewis structure, keep the formal charge to a minimum. In this case, hydrogen has 1 valence electron and it used 1 in bonding, so 1-1=0. Chlorine has 7 valence electrons and it used 1 in a bond with hydrogen and used 6 in lone pairs, 7-1-6=0. Make sure that the octet on each atom has been completed, with the exception of hydrogen which follows the “duet rule,” meaning that it only needs 2 electrons to fill its valence shell. Hydrogen has 2 valence electrons around it, while chlorine has 8 around it so the structure is correct!
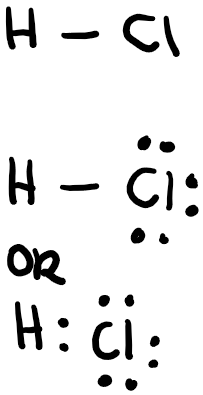
Let’s draw some Lewis structures of other covalent compounds.
Water:

Remember that electrons are of the same charge (-1), and this causes repulsion. So make sure you arrange the electrons in a shape that minimizes repulsion. (This is called the VSEPR theory; valence shell electron pair repulsion). And remember that in real life, molecules are 3d structures, so all atoms don’t have to lie in the same plane if it minimizes repulsion. It has been demonstrated that lone pairs cause more repulsion than bond pairs, which accounts for water’s “bent” shape.
Phosphorus pentachloride
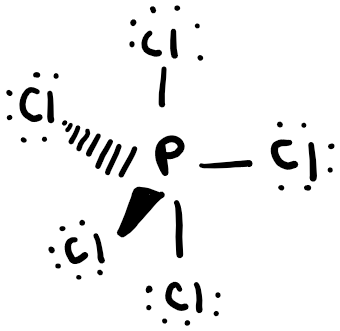
In this case, all atoms do not lie in the same plane. This is shown by the “wedge,” representing a bond line that is out of the plane of the paper, and a “dash,” representing a bond that is behind the plane of the paper.
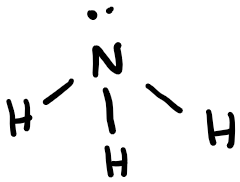
Ammonia
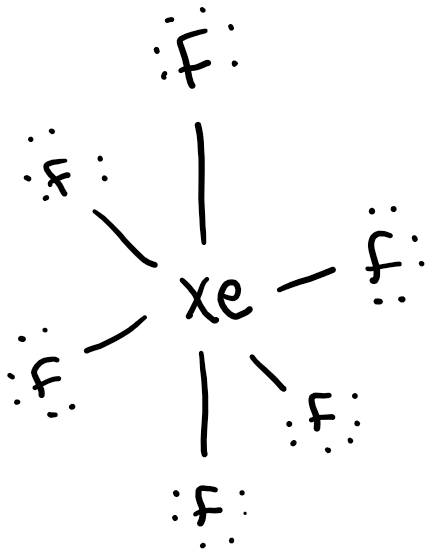
Xenon hexafluoride
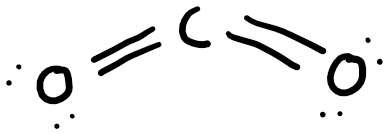
Carbon dioxide