Meaning of Non Polar, Polar Bonds and Electronegativity:
Introduction to Electronegativity part 1 video explains answers to all questions listed below :
- What is a nonpolar bond ?
- What s a polar bond ?
- What is a dipole ?
- Definition and meaning of electronegativity.
- Definition and meaning of electron affinity.
- Difference in electronegativity vs electron affinity ?
Here is the brief summary :
Bond in which there is no charge separation or polarity is called as non polar Bond like hydrogen molecule(H2) or Chlorine molecules (Cl2).
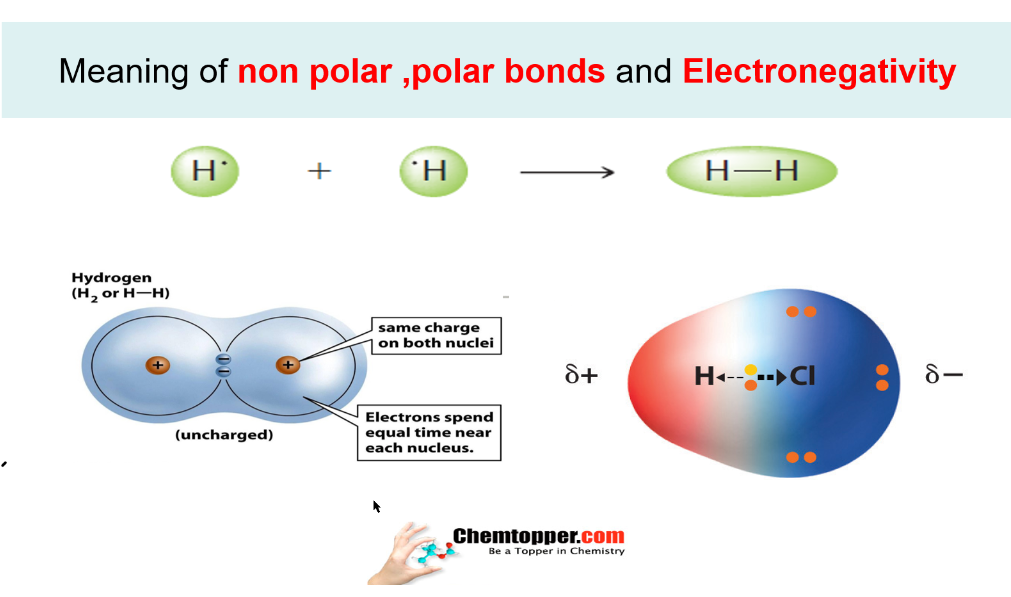
Bond in which there is charge separation or polarity is called as polar bond like HCl or HF molecules.
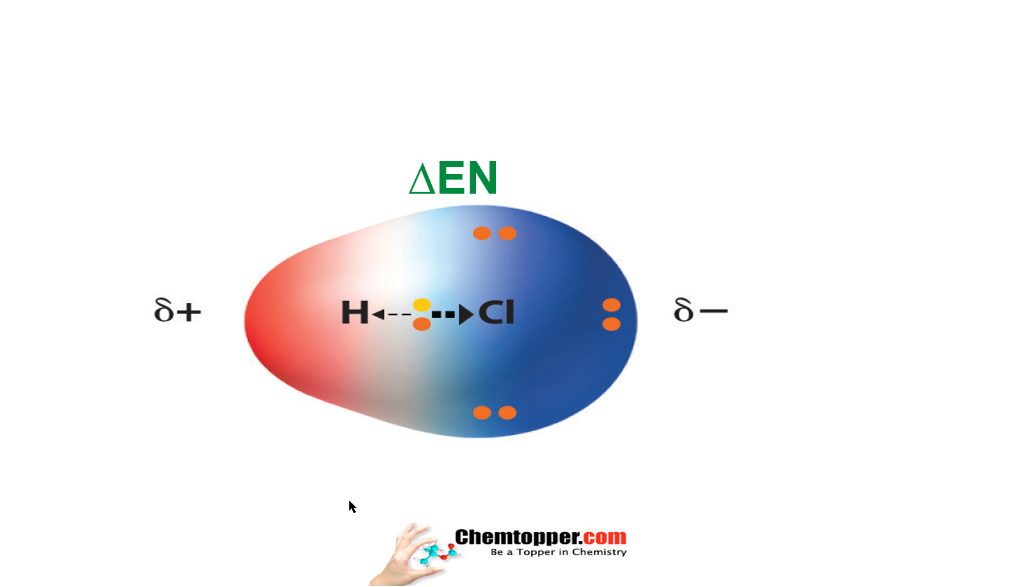
During charge separation due to difference in electronegativity two poles are created in the covalent bond -a partial positive (∂+)and a partial negative (∂-)and is called as a dipole .
Polarity —-> charge separation ——>polar bond ——> dipole
No Polarity—-> No charge separation —->Non polar bond —->No dipole
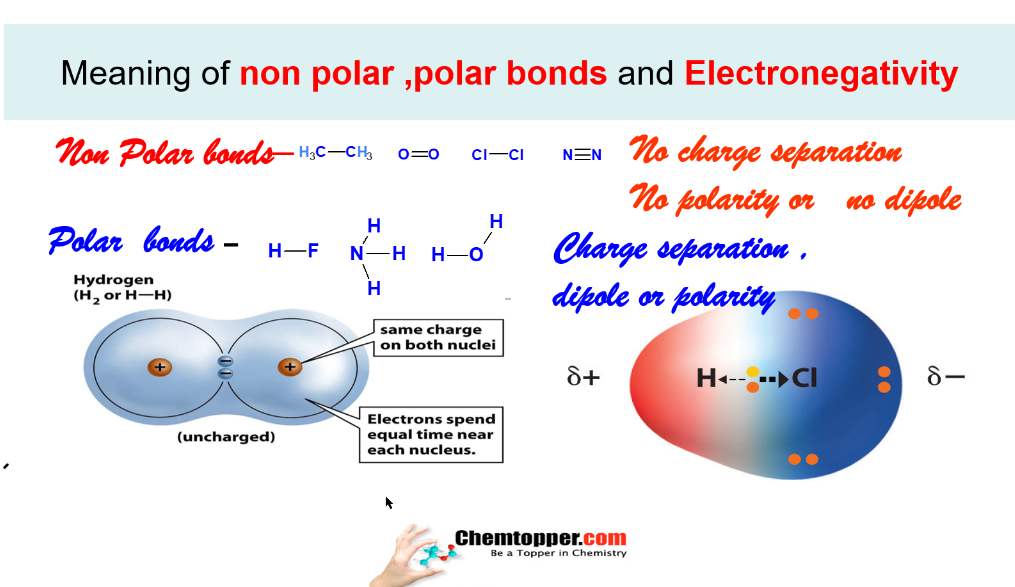
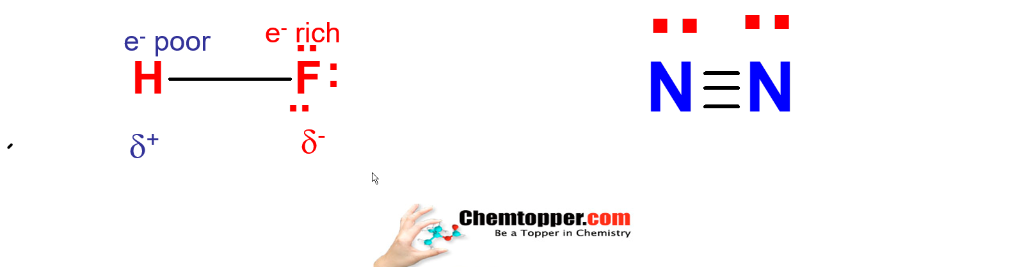
Hydrogen fluorine bond is a polar bond, nitrogen molecule is non polar molecule .
Big Question?? Why is there charge separation in polar bonds?
Answer is difference in electronegativity between the bonded atoms.More is the difference in electronegativity, more is the charge separation and more is the polarity.
In Hydrogen fluoride molecule – fluorine is more electronegative than hydrogen so there is charge separation and bond is polar in nature.
Definition of electronegativity:
Electronegativity is the ability of the tendency of an atom to attract shared electrons on itself in a chemical bond .
Definition of electron affinity:
It is the energy released when an electron is added in an isolated gaseous atom.
Basic difference in EN and EA
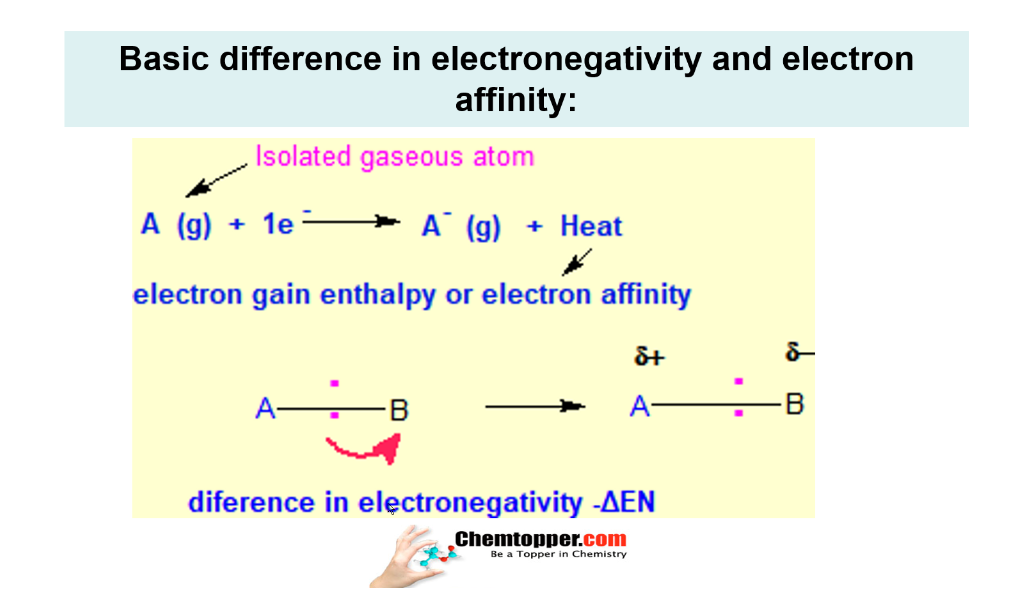
Remember-Electronegativity vs Electron affinity
- Electronegativity is the tendency and not the energy released or required.
- Electronegativity operates in chemical bonds and it is not in isolated atoms.
- Whereas Electron affinity or the electron gain enthalpy is the energy released when an electron is added in the isolated gaseous neutral atom So units of electron gain enthalpy is in terms of joules or kilojoules
- Electronegativity is the tendency so it is not measured in terms of joules or kilo Joule
Introduction to Electronegativity and Electron affinity -Part 1
Chart of electronegativity or periodic table of electronegativity based on Pauling scale:
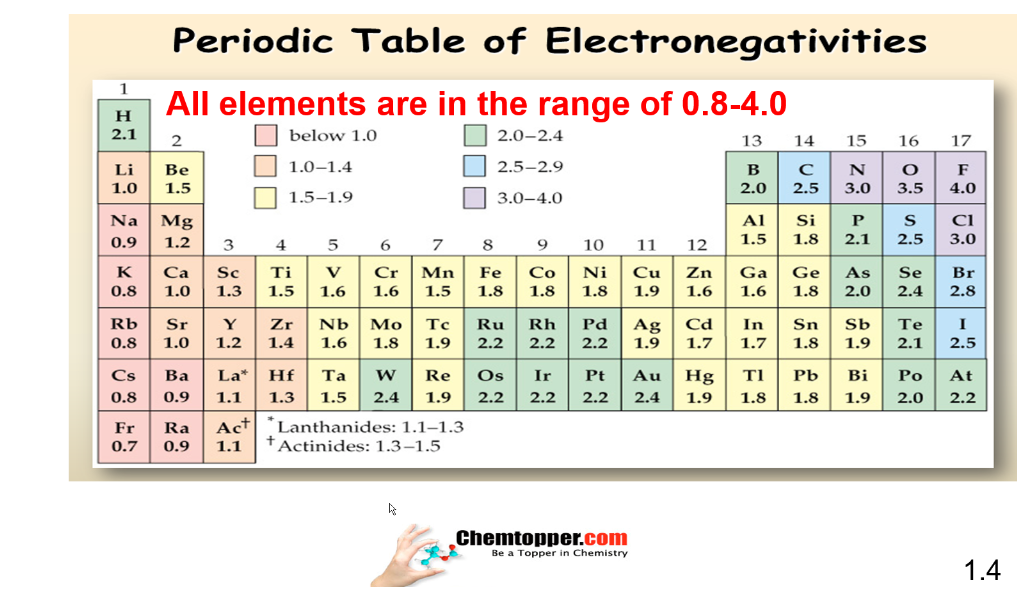
Quiz on Introduction to Electronegativity and Electron affinity -Part 1
Results
#1. Increasing order of electronegativity of few elements based on their position in the periodic table is given here Oxygen> Nitrogen >Sulfur >Carbon >Hydrogen >Boron>Magnesium Use above information and pick up the right option of charge separation in the following chemical bonds
#2. Which carbon is more EN in the following molecule: CH3 – CH+ – CH2 – CH3
#3. Basic difference in EN and EA is
#4. Electronegativity is not measured in in Joules or kilojoules because
#5. Identify the non polar Bond


