|
(1) Sulphur Sulphuric acid Sulphuric acid
S+O2 SO2 SO2
2SO2+O2  2SO3 2SO3
SO3+ H2O  H2SO4 H2SO4
(2) Calcium  Calcium Hydroxide Calcium Hydroxide
2Ca +O2 2CaO 2CaO
CaO+H2O Ca(OH)2 Ca(OH)2
(3) Nitrogen  Nitric Acid (HNO3) + Nitrous Acid (HNO2) Nitric Acid (HNO3) + Nitrous Acid (HNO2)
N2+O2 2NO 2NO
2NO +O 2 2NO2 2NO2
2NO2+H2O  HNO2+HNO3 HNO2+HNO3
(4) Nitrogen  Ammonium Hydroxide Ammonium Hydroxide
N2 NH4OH NH4OH
N2+3H2 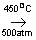 2NH3 2NH3
NH3+H2O NH4OH NH4OH
(5)Sodium  Sodium Hydroxide Sodium Hydroxide
Na  NaOH NaOH
2Na + 2H2O  2NaOH +H2 2NaOH +H2
or 4Na +O2  2Na2O 2Na2O
Na2O+H2O  2 NaOH 2 NaOH
(6) Iron  Ferric Hydroxide Ferric Hydroxide
Fe  Fe(OH) 3 Fe(OH) 3
2Fe+3Cl 2  2FeCl 3 2FeCl 3
FeCl3+3H2O Fe(OH)3+ 3HCl Fe(OH)3+ 3HCl
(7) Iron  Ferrous Hydroxide Ferrous Hydroxide
Fe + 2HCl(cold and dilute) FeCl2 FeCl2
FeCl2+2H2O Fe(OH)2+ 2HCl Fe(OH)2+ 2HCl
(8) Copper  Copper Sulfate Copper Sulfate
Cu + Conc H2SO4  CuSO4 + 2H2O + SO2 CuSO4 + 2H2O + SO2
Chemical reaction to distinguish between Mixture and Comound
Fe +S+2HCl FeCl2+S+H2(blue flame pop sound) FeCl2+S+H2(blue flame pop sound)
FeS+2HCl FeCl2 +H2S( rotten smell gas ) FeCl2 +H2S( rotten smell gas )
Important reactions for the preparation of Oxygen (O2)
Decomposition reactions
Decomposition of red Mercuric oxide
2HgO  2Hg+O2 2Hg+O2 
Decomposition of hydrogen peroxide
2H2O2 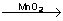 H2+O2 H2+O2
Decomposition of potassium chlorate
2KClO3 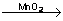 2KCl +3O2 2KCl +3O2
Decomposition of silver(I) oxide
2Ag 2O  4Ag+O2 4Ag+O2 
Decomposition of lead oxide
2Pb 3O4  6PbO 2 6PbO 2 
Red Litharge
2PbO2 2PbO+O 2 2PbO+O 2
Combustion reactions
CxH2y + (x+y/2)O2 xCO2 xCO2 +yH2O +yH2O
(easy way of balancing a combustion reaction of hydrocarbons )
CH4 +2O2  CO2 CO2 +2H2O +2H2O
methane
C2H6 + 7/2O2  2CO2 2CO2 +3H2O +3H2O
ethane
C10H20 + 15O2  10CO2 10CO2 +10H2O +10H2O
decene
C6H14 + 19/2O2  6CO2 6CO2 +7H2O +7H2O
hexane
C2H2+5/2O2  2CO2 2CO2 +H2O +H2O 
ethene
Oxidation of Iron or combustion of iron
4Fe+3O2  2Fe2O3 2Fe2O3
Oxidation of glucose or burning of food
C6H12O6 + 6O2 6CO2 6CO2 +6H2O +6H2O + heat + heat
Reaction of oxygen with nitric oxide
2NO+O2  2NO2 2NO2
Reaction of Magnesium to form magnesium oxide
2Mg + O2 2MgO 2MgO
Formation of Sulfur trioxide or combustion of sulfur trioxide
2SO 2+O2 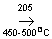 2SO3 2SO3
Oxidation of Ammonia
4NH 3+ 3O 2  2NO2 2NO2 + 6H2O + 6H2O
Oxidation of Aluminium
4Al +3O2  2Al2O3(white) 2Al2O3(white)
Combustion or oxidation of H2S
2H2S+3O2 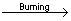 2SO2 2SO2 +2H2O +2H2O
Oxidation of Zinc
2Zn +O2  2ZnO (Yellow when hot white cold) 2ZnO (Yellow when hot white cold)
Oxidation of Carbon disulfide
CS2+3O2  CO2 CO2 +2SO2 +2SO2
Oxidation of lead
Pb+O2  2PbO (Yellow) 2PbO (Yellow)
Oxidation of Zinc sulfide
2ZnS+3O2  2ZnO+2SO2 2ZnO+2SO2
Oxidation of copper
Cu+O2  2CuO (black) 2CuO (black)
Oxidation of mercury
2Hg +O2  2HgO (red) 2HgO (red)
C2H2OH  7O2 7O2 
Catalytic nature of water
4Fe + 3O2 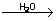 2Fe2O3 2Fe2O3
H2O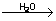 H2 +1/2 O2 H2 +1/2 O2
4P + 5SO2 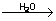 2P2O5 + 5S 2P2O5 + 5S
Example of hydrated Compounds with Common names and Formulae
Barium chloride dihydrate BaCl2 .2H2O
Calcium sulphate dihydrate or Gypsum CaSO2.2H2O
Copper nitrate trihydrate Cu(NO3)2.3H2O
Copper II chloride tetrahydrate CuCl2.4H2O
Copper II sulphate pentrahydrate or Blue vitriol CuSO2.5H2O
Calcium chloride hexahydrate or CaCl2.6H2O
Iron (II) sulphate heptahydrate or Green vitriol FeSO4.7H2 O
Zinc sulphate heptahydrate or White vitriol ZnSO4.7H4O
Magnesium sulphate heptahydrate or Epsom salt MgSO4.7H2O
Sodium carbonate decahydrote or Washing soda Na2CO3.10H2O
Sodium sulphate decahydrate or Glauber’s salt Na2SO4.10H2O
Efflorescence —- When exposed to air lose their water of crystallization and transformed to powder substance .for example Na2CO3.10H2O
Deliquescent crystals —-When exposed to air lose their water of crystallization and transformed to saturated solutions.
For example CuSO4.5H2O ,when exposed to air at ordinary temperature it absorbs water from air and becomes a saturated solution.
CaCl2,6H2O, MgCl2. 6H2O , NaOH, KOH, ZnCl2, Zn (NO3)2,Cu(NO3)2
Hygroscopic Substances absorb moisture without dissolving in water
CaCl2 , MgCl2 , H2SO4,CaO, P2O2, CuO ,silica gel alcohol.
List of some Important Solvents
• S in Carbon DiSulfide CS2
• Paraffin wax in turpentine
• Rubber in benzene
• Iodine in Ethylalcohol
• Paint in turpentine
• Niter in water
• Nail Polish in Acetone
• Rust in oxlalic acid
• Chlorophyll in Methylated sprint
• Oil in Petrol
Flame tests :
As Arsenic Blue Blue
B Boron  Bright green Bright green
Ba Barium  Pale/Apple green Pale/Apple green
Ca Calcium  Brick red Brick red
Cs Caesium  Blue – [Violet (color)Violet] Blue – [Violet (color)Violet]
Cu(I) Copper(I)  Blue Blue
Cu(II) Copper(II) (non-halide)  Green Green
Cu(II) Copper(II) (halide)  Blue-green Blue-green
Fe Iron  Gold Gold
In Indium  Blue Blue
K Potassium [[Lilac (colour)|Lilac](violet] [[Lilac (colour)|Lilac](violet]
Li Lithium  Pink-Red Pink-Red
Mg Magnesium Bright white Bright white
Mo Molybdenum  Yellowish green Yellowish green
Na Sodium  Intense yellow Intense yellow
P Phosphorus  Pale bluish green Pale bluish green
Pb Lead  Blue Blue
Rb Rubidium  Red-violet Red-violet
Sb Antimony  Pale green Pale green
Se Selenium  Azure blue Azure blue
Sr Strontium  Red Red
Te Tellurium  Pale green Pale green
Tl Thallium  Pure green Pure green
Zn Zinc  Bluish green Bluish green
 |
 |
 |
 |
| Copper Compound |
Lithium Compound |
Potassium Compound |
Sodium Compound |
|